Synthesis of Alkenylgold(I) Complexes Relevant to Catalytic Carboxylative Cyclization of Unsaturated Amines and Alcohols
Abstract
:1. Introduction
2. Results and Discussion
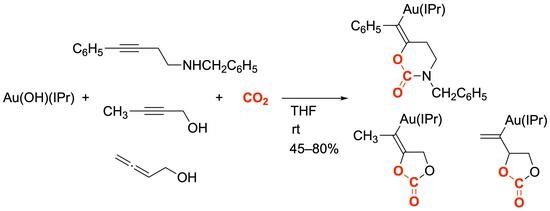
2.1. Synthesis and Characterization of Alkenylgold(I) Complex Derived from Homopropargylamine
2.2. Synthesis and Characterization of Alkenylgold(I) Complex Derived from Propargy Alcohol
2.3. Synthesis and Characterization of Alkenylgold(I) Complex Derived from Allenylmethyl Alcohol
2.4. Protonolysis of Alkenylgold Complexes 7, 9, and 11
3. Materials and Methods
3.1. Synthesis of the Alkenylgold Complexes
3.2. Protonolysis of the Alkenylgold Complexes with Acetic Acid
3.3. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcadi, A. Alternative Synthetic Methods through New Developments in Catalysis by Gold. Chem. Rev. 2008, 108, 3266–3325. [Google Scholar] [CrossRef]
- Debrouwer, W.; Heugebaert, T.S.A.; Roman, B.I.; Stevens, C.V. Homogeneous Gold-Catalyzed Cyclization Reactions of Alkynes with N- and S-Nucleophiles. Adv. Synth. Catal. 2015, 357, 2975–3006. [Google Scholar] [CrossRef]
- Houple, D.B.; Ghorpade, S.; Liu, R.-S. Recent Advances in Gold-Catalyzed N- and O-Functionalizations of Alkynes with Nitrones, Nitroso, Nitro and Nitroxy Species. Adv. Synth. Catal. 2016, 358, 1348–1367. [Google Scholar] [CrossRef]
- Campeau, D.; Rayo, D.F.L.; Mansour, A.; Muratov, K.; Gagosz, F. Gold-Catalyzed Reactions of Specifically Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, T.; Asiri, A.M.; Hashmi, A.S.K. Organometallic Intermediates of Gold Catalysis. Adv. Organomet. Chem. 2014, 62, 261–297. [Google Scholar]
- Liu, L.-P.; Hammond, G.B. Recent advances in the isolation and reactivity of organogold complexes. Chem. Soc. Rev. 2012, 41, 3129–3139. [Google Scholar] [CrossRef] [PubMed]
- Obradors, C.; Echavarren, A.M. Intriguing mechanistic labyrinths in gold(I) catalysis. Chem. Commun. 2014, 50, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Kayaki, Y.; Yamamoto, M.; Suzuki, T.; Ikariya, T. Carboxylative cyclization of propargylamines with supercritical carbon dioxide. Green Chem. 2006, 8, 1019–1021. [Google Scholar] [CrossRef]
- Kayaki, Y.; Mori, N.; Ikariya, T. Palladium-catalyzed carboxylative cyclization of α-allenyl amines in dense carbon dioxide. Tetrahedron Lett. 2009, 50, 6491–6493. [Google Scholar] [CrossRef]
- Hase, S.; Kayaki, Y.; Ikariya, T. NHC–Gold(I) Complexes as Effective Catalysts for the Carboxylative Cyclization of Propargylamines with Carbon Dioxide. Organometallics 2013, 32, 5285–5288. [Google Scholar] [CrossRef]
- Hase, S.; Kayaki, Y.; Ikariya, T. Mechanistic Aspects of the Carboxylative Cyclization of Propargylamines and Carbon Dioxide Catalyzed by Gold(I) Complexes Bearing an N-Heterocyclic Carbene Ligand. ACS Catal. 2015, 5, 5135–5140. [Google Scholar] [CrossRef]
- Yamashita, K.; Hase, S.; Kayaki, Y.; Ikariya, T. Highly Selective Carboxylative Cyclization of Allenylmethylamines with Carbon Dioxide Using N-Heterocyclic Carbene-Silver(I) Catalysts. Org. Lett. 2015, 17, 2334–2337. [Google Scholar] [CrossRef]
- Brunel, P.; Monot, J.; Kefalidis, C.E.; Maron, L.; Martin-Vaca, B.; Bourissou, D. Valorizationof CO2: Preparation of 2-Oxazolidinones by Metal-Ligand Cooperative Catalysis with SCS Indenediide Pd Complexes. ACS Catal. 2017, 7, 2652–2660. [Google Scholar] [CrossRef]
- Wang, W.; Fu, Y.; Li, Y.; Yao, R.; Liu, L.; Chang, W.; Li, J. Ag(I)-Catalyzed solvent-free CO2 capture with homopropargylic amines: An efficient access to 1,3-oxazinan-2-ones. Org. Chem. Front. 2018, 5, 3331–3335. [Google Scholar] [CrossRef]
- Gao, X.-T.; Xie, S.-L.; Zhou, F.; Wu, H.-H.; Zhou, J. Multifunctional 1,3-diphenylguanidine for the carboxylative cyclization of homopropargyl amines with CO2 under ambient temperature and pressure. Chem. Commun. 2019, 55, 14303–14306. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; Ziccarelli, I.; Pomelli, C.S.; Cuocci, C.; Ca’, N.D.; Olivieri, D.; Cartfagna, C.; Gabriele, B. Unexpected cooperative DBU-CuCl2 catalysis for the incorporation of carbon dioxide into homopropargylic amines leading to 6-methylene-1,3-oxazin-2-ones. J. Catal. 2020, 387, 145–153. [Google Scholar] [CrossRef]
- Ghosh, S.; Riyajuddin, S.; Sarkar, S.; Ghosh, K.; Islam, S.M. Pd NPs Decorated on POPs as Recyclable Catalysts for the Synthesis of 2-Oxazolidinones from Propargylic Amines via Atmospheric Cyclizative CO2 Incorporation. ChemNanoMat 2020, 6, 160–172. [Google Scholar] [CrossRef]
- Ghosh, S.; Khan, S.; Ghosh, A.; Chowdhury, A.H.; Haider, M.A.; Khan, A.; Islam, S.M. Utility of Silver Nanoparticles Embedded Covalent Organic Frameworks as Recyclable Catalysts for the Sustainable Synthesis of Cyclic Carbamates and 2-Oxazolidinones via Atmospheric Cyclizative CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 5495–5513. [Google Scholar] [CrossRef]
- Chen, F.; Tao, S.; Deng, Q.-Q.; Wei, D.; Liu, D.B. Binuclear Tridentate Hemilabile Copper(I) Cataysts for Utilization of CO2 into Oxazolidinones from Propargylic Amines. J. Org. Chem. 2020, 85, 15197–15212. [Google Scholar] [CrossRef]
- Dabral, S.; Bayarmagnai, B.; Hermsen, M.; Schießl, J.; Mormul, V.; Hashmi, A.S.K.; Schaub, T. Silver-Catalyzed Carboxylative Cyclization of Primary Propargyl Alcohols with CO2. Org. Lett. 2019, 21, 1422–1425. [Google Scholar] [CrossRef]
- Johnson, C.; Dabral, S.; Rudolf, P.; Licht, U.; Hashmi, A.S.K.; Schaub, T. Liquid-liquid-phase Synthesis of exo-Vinylene Carbonates from Primary Propargyl Alcohols: Catalyst Design and Recycling. ChemCatChem 2021, 13, 353–361. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; Farshadfar, K.; Rudolph, M.; Dabral, S.; Rudolf, P.; Rominger, F.; Schaub, T.; Ariafard, A.; Hashmi, A.S.K. Copper-catalysed synthesis of α-alkylidene cyclic carbonates from propargylic alcohols and CO2. Green Chem. 2021, 23, 889–897. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; Saxl, T.; Stein, P.M.; Rudolph, M.; Dabral, S.; Rudolf, P.; Rominger, F.; Asiri, A.M.; Hashmi, A.S.K. Expanded Ring NHC Silver Carboxylate Complexes as Efficient and Reusable Catalysts for the Carboxylative Cyclization of Unsubstituted Propargylic Derivatives. ChemSusChem 2021, 14, 2367–2374. [Google Scholar] [CrossRef]
- Uemura, K.; Shiraishi, D.; Noziri, M.; Inoue, Y. Preparation of Cyclic Carbonates from Alkadienols, CO2, and Aryl or Vinylic Halides Catalyzed by a Palladium Complex. Bull. Chem. Soc. Jpn. 1999, 72, 1063–1069. [Google Scholar] [CrossRef]
- Wang, W.; Hammond, G.B.; Xu, B. Ligand Effects and Ligand Design in Homogeneous Gold(I) Catalysis. J. Am. Chem. Soc. 2012, 134, 5697–5705. [Google Scholar] [CrossRef]
- BabaAhmadi, R.; Ghanbari, P.; Rajabi, N.A.; Hashmi, A.S.K.; Yates, B.F.; Ariafard, A. A Theoretical Study on the Protodeauration Step of the Gold(I)-Catalyzed Organic Reactions. Organometallics 2015, 34, 3186–3195. [Google Scholar] [CrossRef]
- Jo, T.; Taschinski, S.; Leach, I.F.; Bauer, C.; Hashmi, A.S.K.; Klein, J.E.M.N. On the Role of Noncovalent Ligand-Substrate Interactions in Au(I) Catalysis: An Experimental and Computational Study of Protodeauration. ACS Catal. 2022, 12, 13158–13163. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.; Burton, J.W. Palladium-Catalysed Cyclisation of N-Alkynyl Aminomalonates. Chem. Eur. J. 2010, 16, 12303–12306. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Ma, S. CuI-Catalyzed Synthesis of Functionalized Terminal Allenes from 1-Alkynes. Eur. J. Org. Chem. 2013, 3041–3048. [Google Scholar] [CrossRef]
- Gómez-Suárez, A.; Ramón, R.S.; Slawin, A.M.Z.; Nolan, S.P. Synthetic routes to [Au(NHC)(OH)] (NHC = N-heterocyclic carbene) complexes. Dalton Trans. 2012, 41, 5461–5463. [Google Scholar] [CrossRef] [PubMed]
- CrystalStructure 4.1: Crystal Structure Analysis Package, Version 4.1.; Rigaku Coorporation: Tokyo, Japan, 2015.
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hase, S.; Yamashita, K.; Kayaki, Y. Synthesis of Alkenylgold(I) Complexes Relevant to Catalytic Carboxylative Cyclization of Unsaturated Amines and Alcohols. Molecules 2024, 29, 1331. https://doi.org/10.3390/molecules29061331
Hase S, Yamashita K, Kayaki Y. Synthesis of Alkenylgold(I) Complexes Relevant to Catalytic Carboxylative Cyclization of Unsaturated Amines and Alcohols. Molecules. 2024; 29(6):1331. https://doi.org/10.3390/molecules29061331
Chicago/Turabian StyleHase, Shun, Kyohei Yamashita, and Yoshihito Kayaki. 2024. "Synthesis of Alkenylgold(I) Complexes Relevant to Catalytic Carboxylative Cyclization of Unsaturated Amines and Alcohols" Molecules 29, no. 6: 1331. https://doi.org/10.3390/molecules29061331