The Effect of Ultrasound on the Extraction and Functionality of Proteins from Duckweed (Lemna minor)
Abstract
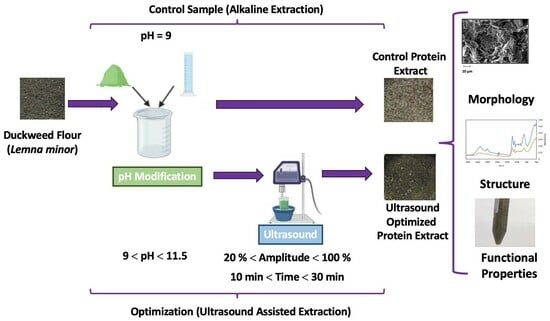
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis of Duckweed (Lemna minor)
2.2. Optimization of the Extraction of Protein from Duckweed
2.3. Evaluation of the Effect of Ultrasound on the Alkaline Extraction of Protein from Duckweed (Lemna minor)
2.3.1. Morphological and Structural Effects of Ultrasound on the Extracted Protein
2.3.2. Functional Properties of the Duckweed Flour and Its Extracted Protein
3. Materials and Methods
3.1. Raw Material and Proximate Analysis
3.2. Optimization of the Alkaline Extraction of Protein Assisted with Ultrasound through a Box-Behnken Design of Experiments (DOE)
3.3. Comparison of the Effect of Ultrasound in the Alkaline Extraction of Protein
3.3.1. Colorimetric Analysis of the Samples
3.3.2. Scanning Electron Microscopy (SEM) of the Samples
3.3.3. Fourier Transformed Infrared Spectroscopy (FTIR) of the Samples
3.3.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.3.5. Functional Properties of the Obtained Samples
Water Solubility Index (%) and Water Absorption Index
Foaming Activity (%), Foam Density (%), and Foam Stability (%)
Emulsifying Capacity and Emulsifying Stability
3.4. DOE Analysis and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortinau, L.C.; Hoertel, H.A.; Douglas, S.M.; Leidy, H.J. Effects of high-protein vs. high-fat snacks on appetite control, satiety, and eating initiation in healthy women. Nutr. J. 2014, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Liu, Y.; Iizuka, K.; Kuwata, H.; Seino, Y.; Yabe, D. A review of recent findings on meal sequence: An attractive dietary approach to prevention and management of type 2 diabetes. Nutrients 2020, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Protein intake and risk of type 2 diabetes in US men and women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Zhao, L.G.; Zhang, Q.L.; Liu, X.L.; Wu, H.; Zheng, J.L.; Xiang, Y.B. Dietary protein intake and risk of type 2 diabetes: A dose–response meta-analysis of prospective studies. Eur. J. Nutr. 2019, 58, 1351–1367. [Google Scholar] [CrossRef]
- Qi, X.X.; Shen, P. Associations of dietary protein intake with all-cause, cardiovascular disease, and cancer mortality: A systematic review and meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, A.; Okamura, T.; Sugiyama, D.; Higashiyama, A.; Watanabe, M.; Okuda, N.; Kadota, A.; Miyagawa, N.; Fujiyoshi, A.; Yoshita, K.; et al. Vegetable protein intake was inversely associated with cardiovascular mortality in a 15-year follow-up study of the general Japanese population. J. Atheroscler. Thromb. 2019, 26, 198–206. [Google Scholar] [CrossRef]
- Miki, A.; Hashimoto, Y.; Matsumoto, S.; Ushigome, E.; Fukuda, T.; Sennmaru, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. Protein intake, especially vegetable protein intake, is associated with higher skeletal muscle mass in elderly patients with type 2 diabetes. J. Diabetes Res. 2017, 2017, 7985728. [Google Scholar] [CrossRef]
- Ouyang, Y.; Huang, F.; Zhang, X.; Li, L.; Zhang, B.; Wang, Z.; Wang, H. Association of dietary protein intake with muscle mass in elderly Chinese: A cross-sectional study. Nutrients 2022, 14, 5130. [Google Scholar] [CrossRef]
- Carballo-Casla, A.; Sotos-Prieto, M.; García-Esquinas, E.; Struijk, E.A.; Caballero, F.F.; Calderón-Larrañaga, A.; Lopez-Garcia, E.; Rodríguez-Artalejo, F.; Ortolá, R. Animal and vegetable protein intake and malnutrition in older adults: A multicohort study. J. Nutr. Health Aging 2023, 28, 100002. [Google Scholar] [CrossRef]
- Ortolá, R.; Struijk, E.A.; García-Esquinas, E.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Changes in dietary intake of animal and vegetable protein and unhealthy aging. Am. J. Med. 2020, 133, 231–239. [Google Scholar] [CrossRef]
- Zhubi-Bakija, F.; Bajraktari, G.; Bytyçi, I.; Mikhailidis, D.P.; Henein, M.Y.; Latkovskis, G.; Rexhaj, Z.; Zhubi, E.; Zirlik, A. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP). Clin. Nutr. 2021, 40, 255–276. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins–Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef]
- Bryant, C.J. Plant-based animal product alternatives are healthier and more environmentally sustainable than animal products. Future Foods 2022, 6, 100174. [Google Scholar] [CrossRef]
- Xu, J.; Shen, Y.; Zheng, Y.; Smith, G.; Sun, X.S.; Wang, D.; Zhao, Y.; Li, Y. Duckweed (Lemnaceae) for potentially nutritious human food: A review. Food Rev. Int. 2023, 39, 3620–3634. [Google Scholar] [CrossRef]
- Sońta, M.; Rekiel, A.; Batorska, M. Use of duckweed (Lemna L.) in sustainable livestock production and aquaculture—A review. Ann. Anim. Sci. 2019, 19, 257–271. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Lemna minor: Traditional uses, chemical constituents and pharmacological effects—A review. IOSR J. Pharm. 2019, 9, 6–11. [Google Scholar]
- Iatrou, E.I.; Kora, E.; Stasinakis, A.S. Investigation of biomass production, crude protein and starch content in laboratory wastewater treatment systems planted with Lemna minor and Lemna gibba. Environ. Technol. 2018, 40, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Akyurt, İ.; Günal, G. Use of duckweed, Lemna minor, as a protein feedstuff in practical diets for common carp, Cyprinus carpio, fry. Turk. J. Fish. Aquat. Sci. 2004, 4, 105–109. [Google Scholar]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. Process Intensif. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Das, R.S.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing in alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochem. 2022, 91, 106234. [Google Scholar]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Su, J.; Cavaco-Paulo, A. Effect of ultrasound on protein functionality. Ultrason. Sonochem. 2021, 76, 105653. [Google Scholar] [CrossRef]
- Omura, M.H.; de Oliveira, A.P.H.; de Souza Soares, L.; dos Reis Coimbra, J.S.; de Barros, F.A.R.; Vidigal, M.C.T.R.; Baracat-Pereira, M.C.; de Oliveira, E.B. Effects of protein concentration during ultrasonic processing on physicochemical properties and techno-functionality of plant food proteins. Food Hydrocoll. 2021, 113, 106457. [Google Scholar] [CrossRef]
- O’sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef]
- Shokri, S.; Javanmardi, F.; Mohammadi, M.; Khaneghah, A.M. Effects of ultrasound on the techno-functional properties of milk proteins: A systematic review. Ultrason. Sonochem. 2022, 83, 105938. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.; Ngadi, M. Ultrasound-assisted processing: Science, technology and challenges for the plant-based protein industry. Ultrason. Sonochem. 2022, 84, 105955. [Google Scholar] [CrossRef]
- Sengar, A.S.; Thirunavookarasu, N.; Choudhary, P.; Naik, M.; Surekha, A.; Sunil, C.K.; Rawson, A. Application of power ultrasound for plant protein extraction, modification and allergen reduction—A review. Appl. Food Res. 2022, 2, 100219. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: Effects in yield and functional properties of protein isolates. Food Bioprocess Technol. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Engelen-Smit, N.P.; Van Der Meer, I.M.; America, A.H.; Mes, J.J.; Kootstra, A.M.J.; Henket, J.T.M.M.; Mulder, W.J. Isolation and gelling properties of duckweed protein concentrate. ACS Food Sci. Technol. 2021, 1, 908–916. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Bioenergy potential of duckweed (Lemna gibba L.) biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2231–2237. [Google Scholar]
- Hu, Z.; Fang, Y.; Yi, Z.; Tian, X.; Li, J.; Jin, Y.; He, K.; Liu, P.; Du, A.; Huang, Y.; et al. Determining the nutritional value and antioxidant capacity of duckweed (Wolffia arrhiza) under artificial conditions. LWT 2022, 153, 112477. [Google Scholar] [CrossRef]
- Sharma, J.; Clark, W.D.; Shrivastav, A.K.; Goswami, R.K.; Tocher, D.R.; Chakrabarti, R. Production potential of greater duckweed Spirodela polyrhiza (L. Schleiden) and its biochemical composition evaluation. Aquaculture 2019, 513, 734419. [Google Scholar] [CrossRef]
- Muller, T.; Bernier, M.È.; Bazinet, L. Optimization of water lentil (duckweed) leaf protein purification: Identification, structure, and foaming properties. Foods 2023, 12, 3424. [Google Scholar] [CrossRef]
- Duangjarus, N.; Chaiworapuek, W.; Rachtanapun, C.; Ritthiruangdej, P.; Charoensiddhi, S. Antimicrobial and functional properties of duckweed (Wolffia globosa) protein and peptide extracts prepared by ultrasound-assisted extraction. Foods 2022, 11, 2348. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Duckweed: An effective tool for phyto-remediation. Toxicol. Environ. Chem. 2013, 95, 1256–1266. [Google Scholar] [CrossRef]
- Ifie, I.; Olatunde, S.; Ogbon, O.; Umukoro, J.E. Processing techniques on phytochemical content, proximate composition, and toxic components in duckweed. Int. J. Veg. Sci. 2021, 27, 294–302. [Google Scholar] [CrossRef]
- Fourounjian, P.; Fakhoorian, T.; Cao, X.H. Importance of duckweeds in basic research and their industrial applications. In Duckweed Genomes; Springer: Cham, Switzerland, 2020; pp. 1–17. [Google Scholar]
- Devlamynck, R.; de Souza, M.F.; Bog, M.; Leenknegt, J.; Eeckhout, M.; Meers, E. Effect of the growth medium composition on nitrate accumulation in the novel protein crop Lemna minor. Ecotoxicol. Environ. Saf. 2020, 206, 111380. [Google Scholar] [CrossRef] [PubMed]
- Siriwat, W.; Ungwiwatkul, S.; Unban, K.; Laokuldilok, T.; Klunklin, W.; Tangjaidee, P.; Potikanond, S.; Kaur, L.; Phongthai, S. Extraction, enzymatic modification, and anti-cancer potential of an alternative plant-based protein from Wolffia globosa. Foods 2023, 12, 3815. [Google Scholar] [CrossRef]
- Dukić, J.; Košpić, K.; Kelava, V.; Mavrić, R.; Nutrizio, M.; Balen, B.; Butorac, A.; Öztop, M.H.; Jambrak, A.R. Alternative methods for RuBisCO extraction from sugar beet waste: A comparative approach of ultrasound and high voltage electrical discharge. Ultrason. Sonochem. 2023, 99, 106535. [Google Scholar] [CrossRef]
- Chandra, R.D.; Prihastyanti, M.N.U.; Lukitasari, D.M. Effects of pH, high pressure processing, and ultraviolet light on carotenoids, chlorophylls, and anthocyanins of fresh fruit and vegetable juices. eFood 2021, 2, 113–124. [Google Scholar] [CrossRef]
- Yoksan, R.; Boontanimitr, A.; Klompong, N.; Phothongsurakun, T. Poly (lactic acid)/thermoplastic cassava starch blends filled with duckweed biomass. Int. J. Biol. Macromol. 2022, 203, 369–378. [Google Scholar] [CrossRef]
- Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella vulgaris residue after lipid separation using response surface methodology. J. Food Sci. Technol. 2014, 51, 2006–2013. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Kumar, G.; Upadhyay, S.; Yadav, D.K.; Malakar, S.; Dhurve, P.; Suri, S. Application of ultrasound technology for extraction of color pigments from plant sources and their potential bio-functional properties: A review. J. Food Process Eng. 2023, 46, e14238. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams III, W.W.; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Inguanez, L.; Zhu, X.; de Oliveira Mallia, J.; Tiwari, B.K.; Valdramidis, V.P. Extractions of protein-rich Alaria esculenta and Lemna minor by the use of high-power (assisted) ultrasound. Sustainability 2023, 15, 8024. [Google Scholar] [CrossRef]
- Hildebrand, G.; Poojary, M.M.; O’Donnell, C.; Lund, M.N.; Garcia-Vaquero, M.; Tiwari, B.K. Ultrasound-assisted processing of Chlorella vulgaris for enhanced protein extraction. J. Appl. Phycol. 2020, 32, 1709–1718. [Google Scholar] [CrossRef]
- Hu, L.X.; Tian, F.; Martin, F.L.; Ying, G.G. Biochemical alterations in duckweed and algae induced by carrier solvents: Selection of an appropriate solvent in toxicity testing. Environ. Toxicol. Chem. 2017, 36, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, H.; Venkateshan, K.; Yan, S.; Cheng, J.; Sun, X.S.; Wang, D. Functional, physiochemical, and rheological properties of duckweed (Spirodela polyrhiza) protein. Trans. ASABE 2011, 54, 555–561. [Google Scholar] [CrossRef]
- Bharti, R.K.; Srivastava, S.; Thakur, I.S. Proteomic analysis of carbon concentrating chemolithotrophic bacteria Serratia sp. for sequestration of carbon dioxide. PLoS ONE 2014, 9, e91300. [Google Scholar] [CrossRef]
- Liebers, M.; Hommel, E.; Grübler, B.; Danehl, J.; Offermann, S.; Pfannschmidt, T. Photosynthesis in the biomass model species Lemna minor displays plant-conserved and species-specific features. Plants 2023, 12, 2442. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yang, R.; Niu, L.; Wang, W. Comparison of protein extraction methods for 2DE-based proteomic analysis of duckweed Spirodela polyrhiza, a small aquatic model plant. Aquat. Bot. 2020, 163, 103216. [Google Scholar] [CrossRef]
- Soria-Hernández, C.; Serna-Saldívar, S.; Chuck-Hernández, C. Physicochemical and functional properties of vegetable and cereal proteins as potential sources of novel food ingredients. Food Technol. Biotechnol. 2015, 53, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C. Determination of fat content. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 29–37. [Google Scholar]
- AOAC. Method 925.10. Solids (total) and moisture in flour. In Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Method 923.03. Ash of flour. In Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Rakha, A.; Anjum, F.M.; Ahmed, W.; Sohail, M. Effects of extrusion cooking on the dietary fibre content and Water Solubility Index of wheat bran extrudates. Int. J. Food Sci. Technol. 2015, 50, 1533–1537. [Google Scholar] [CrossRef]
- Yousf, N.; Nazir, F.; Salim, R.; Ahsan, H.; Sirwal, A. Water solubility index and water absorption index of extruded product from rice and carrot blend. J. Pharmacogn. Phytochem. 2017, 6, 2165–2168. [Google Scholar]
- Haque, Z.; Kito, M. Lipophilization of. alpha. s1-casein. 2. Conformational and functional effects. J. Agric. Food Chem. 1983, 31, 1231–1237. [Google Scholar] [CrossRef]
- Killekar, V.C.; Koli, J.M.; Sharangdhar, S.T.; Metar, S.Y. Functional properties of gelatin extracted from skin of black kingfish (Ranchycentron canadus). Indian J. Fundam. Appl. Life Sci. 2012, 2, 106–116. [Google Scholar]





| Duckweed Specie * | Moisture (%, WB) | Fat (%, DW) | Protein (%, DW) | Ash (%, DW) | Total Carbohydrates ** (%, DW) | Reference |
|---|---|---|---|---|---|---|
| Lemna minor | 6.23 ± 0.13 | 1.32 ± 0.10 | 21.74 ± 0.31 | 14.71 ± 0.08 | ND | This work |
| Lemna minor | NR | 4–4.4 | 16–45 | 4–5 | NR | [16] |
| Lemna gibba (FD) | 93.7 (F) | 3.4 | 33.6 | 18 | 28.8 | [29] |
| Lemna gibba L. (B) | 10.03 | 7.61 | 22.72 | 13.23 | 36.23 | [30] |
| Wolffia arrhizal (F) | 95.18 | 6.07 | 50.89 | 11.71 | 31.3 | [31] |
| Spirodela polyrhiza (F) | NR | 7.11 | 35.82 | 18.51 | 38.38 | [32] |
| Duckweed (DD) | 3.7 | NR | 35.8 | 6.2 | 47.2 | [33] |
| Wolffia globosa (D) | 8.61 | 3.03 | 33.16 | 14.58 | 49.22 | [34] |
| Lemna minor | NR | 4.75 | 28.48 | 10.1 | NR | [35] |
| Lemna spp. | NR | 4.5–9.8 | 36.0–38.6 | 8.46–19.0 | NR | [35] |
| Duckweed (SD) | NR | 2.23 | 27.67 | 12.63 | NR | [36] |
| pH | Amplitude (%) | Time (min) | SY 1 (%) | PC 1 (%) | PEY 1 (%) |
|---|---|---|---|---|---|
| 14.0 | 60 | 10 | 85.08 ± 7.99 | 14.60 ± 0.66 | 61.03 ± 8.47 |
| 14.0 | 100 | 20 | 79.62 ± 7.48 | 13.63 ± 0.31 | 54.88 ± 6.23 |
| 11.5 | 60 | 20 | 13.91 ± 4.20 | 38.73 ± 1.39 | 26.26 ± 7.03 |
| 9.0 | 60 | 30 | 15.06 ± 4.01 | 37.28 ± 6.37 | 26.90 ± 2.62 |
| 11.5 | 20 | 10 | 16.47 ± 1.32 | 35.74 ± 2.49 | 28.92 ± 4.33 |
| 14.0 | 20 | 20 | 73.69 ± 11.67 | 21.90 ± 8.90 | 41.46 ± 12.17 |
| 11.5 | 20 | 30 | 13.64 ± 2.39 | 38.44 ± 3.97 | 25.47 ± 1.84 |
| 11.5 | 100 | 10 | 17.74 ± 2.53 | 36.97 ± 3.69 | 31.91 ± 1.37 |
| 9.0 | 100 | 20 | 12.42 ± 1.67 | 36.79 ± 6.08 | 22.14 ± 0.70 |
| 11.5 | 100 | 30 | 16.05 ± 2.76 | 42.17 ± 1.84 | 33.30 ± 7.15 |
| 14.0 | 60 | 30 | 46.14 ± 5.11 | 21.22 ± 0.12 | 47.98 ± 5.04 |
| 11.5 | 60 | 20 | 22.08 ± 4.99 | 31.96 ± 2.05 | 34.34 ± 5.59 |
| 9.0 | 60 | 10 | 13.47 ± 2.34 | 36.70 ± 0.47 | 24.25 ± 4.52 |
| 9.0 | 20 | 20 | 13.74 ± 1.13 | 39.31 ± 3.40 | 24.89 ± 2.09 |
| 11.5 | 60 | 20 | 16.54 ± 2.05 | 40.72 ± 3.39 | 32.85 ± 1.34 |
| Response | Regression Equation | r2 |
|---|---|---|
| Solids Yield (%) | = 348.3 − 75.92 pH − 0.324 Amplitude + 5.39 Time + 4.106 pH × pH + 0.00106 Amplitude × Amplitude − 0.0323 Time × Time + 0.0181 pH × Amplitude − 0.4054 pH × Time + 0.00071 Amplitude × Time | 95.77 |
| Protein Content (%) | = −123.8 + 32.72 pH + 0.054 Amplitude − 0.747 Time − 1.609 pH × pH + 0.000516 Amplitude × Amplitude + 0.0037 Time × Time − 0.0144 pH × Amplitude + 0.0604 pH × Time + 0.00156 Amplitude × Time | 86.66 |
| Protein Yield (%) | = 120.8 − 21.22 pH − 0.254 Amplitude + 0.88 Time + 1.187 pH × pH − 0.00170 Amplitude × Amplitude + 0.0148 Time × Time + 0.0404 pH × Amplitude − 0.1570 pH × Time + 0.00302 Amplitude × Time | 86.65 |
| Condition | pH | Amplitude | Time | SY (%) | PC (%) | PEY (%) | |
|---|---|---|---|---|---|---|---|
| Optimal Point | 11.5 | 60 | 20 | Predicted | 17.51 | 37.14 | 31.15 |
| Real | 14.86 ± 0.26 | 41.30 ± 1.24 | 30.08 ± 0.90 | ||||
| %Fit | 84.87 | 111.20 | 96.57 |
| Sample | L* | a* | b* | Appearance | RGB Space |
|---|---|---|---|---|---|
| Duckweed Flour | 47.32 ± 0.75 a | 4.04 ± 0.13 b | 35.60 ± 0.45 c |  |  |
| Control Protein Extract | 45.66 ± 1.32 a | 4.51 ± 0.10 b | 39.06 ± 0.11 b |  |  |
| Ultrasound Protein Extract | 24.41 ± 0.48 b | 9.64 ± 0.05 a | 79.04 ± 0.64 a |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirón-Mérida, V.A.; Soria-Hernández, C.; Richards-Chávez, A.; Ochoa-García, J.C.; Rodríguez-López, J.L.; Chuck-Hernández, C. The Effect of Ultrasound on the Extraction and Functionality of Proteins from Duckweed (Lemna minor). Molecules 2024, 29, 1122. https://doi.org/10.3390/molecules29051122
Mirón-Mérida VA, Soria-Hernández C, Richards-Chávez A, Ochoa-García JC, Rodríguez-López JL, Chuck-Hernández C. The Effect of Ultrasound on the Extraction and Functionality of Proteins from Duckweed (Lemna minor). Molecules. 2024; 29(5):1122. https://doi.org/10.3390/molecules29051122
Chicago/Turabian StyleMirón-Mérida, Vicente Antonio, Cintya Soria-Hernández, Alejandro Richards-Chávez, Juan Carlos Ochoa-García, Jorge Luis Rodríguez-López, and Cristina Chuck-Hernández. 2024. "The Effect of Ultrasound on the Extraction and Functionality of Proteins from Duckweed (Lemna minor)" Molecules 29, no. 5: 1122. https://doi.org/10.3390/molecules29051122