Organosolv Treatment of Red Grape Pomace for Effective Recovery of Antioxidant Polyphenols and Pigments Using a Ternary Glycerol/Ethanol/Water System under Mild Acidic Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experimentation
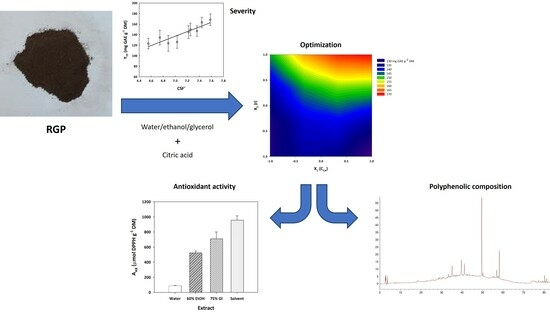
2.2. Severity-Based Modeling
2.3. Response Surface-Based Modeling
2.4. Assessment of Treatment Performance
2.5. Effect on Polyphenolic Composition and Antioxidant Characteristics
3. Materials and Methods
3.1. Chemicals
3.2. Red Grape Pomace (RGP)
3.3. Extraction Procedures
3.4. Determination of Treatment Severity
3.5. Experimental Design for Treatment Optimization
3.6. Analyses for Total Polyphenols, Total Pigments, Total Flavanols and Antioxidant Properties
3.7. Chromatographic Analyses
3.8. Data Handling and Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imbert, E. Food waste valorization options: Opportunities from the bioeconomy. Open Agric. 2017, 2, 195–204. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Rodrigues Machado, A.; Atatoprak, T.; Santos, J.; Alexandre, E.M.; Pintado, M.E.; Paiva, J.A.; Nunes, J. Potentialities of the Extraction Technologies and Use of Bioactive Compounds from Winery By-Products: A Review from a Circular Bioeconomy Perspective. Appl. Sci. 2023, 13, 7754. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.M.P.; Leos, M.D.S.; Solis, V.E.; Domínguez, J.M. Recent trends on the valorization of winemaking industry wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100415. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y. Bioactive compounds, health benefits and food applications of grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: A review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Wani, T.A.; Majid, D.; Dar, B.; Makroo, H.A.; Allai, F.M. Utilization of novel techniques in extraction of polyphenols from grape pomace and their therapeutic potential: A review. J. Food Meas. Charact. 2023, 17, 5412–5425. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Makris, D.P.; Lalas, S. Glycerol and glycerol-based deep eutectic mixtures as emerging green solvents for polyphenol extraction: The evidence so far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Bondancia, T.J.; de Aguiar, J.; Batista, G.; Cruz, A.J.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of nanocellulose using citric acid in a biorefinery concept: Effect of the hydrolysis reaction time and techno-economic analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly efficient and sustainable preparation of carboxylic and thermostable cellulose nanocrystals via FeCl3-catalyzed innocuous citric acid hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Gao, Y.; Zietsman, A.J.; Vivier, M.A.; Moore, J.P. Deconstructing wine grape cell walls with enzymes during winemaking: New insights from glycan microarray technology. Molecules 2019, 24, 165. [Google Scholar] [CrossRef]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem. Eng. Com. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotech. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 2014, 42786. [Google Scholar] [CrossRef]
- Garcia-Montalvo, J.; Garcia-Martín, A.; Ibañez Bujan, J.; Santos Mazorra, V.E.; Yustos Cuesta, P.; Bolivar, J.M.; Ladero, M. Extraction of antioxidants from grape and apple pomace: Solvent selection and process kinetics. Appl. Sci. 2022, 12, 4901. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.; Teles, A.S.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.; Cabral, L.M.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhaes, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Filippi, K.; Papapostolou, H.; Alexandri, M.; Vlysidis, A.; Myrtsi, E.D.; Ladakis, D.; Pateraki, C.; Haroutounian, S.A.; Koutinas, A. Integrated biorefinery development using winery waste streams for the production of bacterial cellulose, succinic acid and value-added fractions. Bioresour. Technol. 2022, 343, 125989. [Google Scholar] [CrossRef]
- Xu, Y.; Burton, S.; Kim, C.; Sismour, E. Phenolic compounds, antioxidant, and antibacterial properties of pomace extracts from four Virginia-grown grape varieties. Food Sci. Nutr. 2016, 4, 125–133. [Google Scholar] [CrossRef]
- Ravindran, R.; Desmond, C.; Jaiswal, S.; Jaiswal, A.K. Optimisation of organosolv pretreatment for the extraction of polyphenols from spent coffee waste and subsequent recovery of fermentable sugars. Bioresour. Technol. Rep. 2018, 3, 7–14. [Google Scholar] [CrossRef]
- Chotirotsukon, C.; Raita, M.; Champreda, V.; Laosiripojana, N. Fractionation of sugarcane trash by oxalic-acid catalyzed glycerol-based organosolv followed by mild solvent delignification. Ind. Crops Prod 2019, 141, 111753. [Google Scholar] [CrossRef]
- Chotirotsukon, C.; Raita, M.; Yamada, M.; Nishimura, H.; Watanabe, T.; Laosiripojana, N.; Champreda, V. Sequential fractionation of sugarcane bagasse using liquid hot water and formic acid-catalyzed glycerol-based organosolv with solvent recycling. BioEnergy Res. 2021, 14, 135–152. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Dragović-Uzelac, V. Optimizing acidity and extraction time for polyphenolic recovery and antioxidant capacity in grape pomace skin extracts with response surface methodology approach. J. Food Process. Preserv. 2016, 40, 1256–1263. [Google Scholar] [CrossRef]
- Brazinha, C.; Cadima, M.; Crespo, J.G. Optimization of extraction of bioactive compounds from different types of grape pomace produced at wineries and distilleries. J. Food Sci. 2014, 79, E1142–E1149. [Google Scholar] [CrossRef] [PubMed]
- Metivier, R.; Francis, F.; Clydesdale, F. Solvent extraction of anthocyanins from wine pomace. J. Food Sci. 1980, 45, 1099–1100. [Google Scholar] [CrossRef]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Kinetic modelling for flavanol extraction from red grape (Vitis vinifera L.) pomace using aqueous organic acid solutions. Int. Food Res. J. 2014, 21, 1919. [Google Scholar]
- Harsha, P.S.; Gardana, C.; Simonetti, P.; Spigno, G.; Lavelli, V. Characterization of phenolics, in vitro reducing capacity and anti-glycation activity of red grape skins recovered from winemaking by-products. Bioresour. Technol 2013, 140, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Natolino, A. Optimization of the extraction of phenolic compounds from red grape marc (Vitis vinifera L.) using response surface methodology. J. Wine Res. 2018, 29, 26–36. [Google Scholar] [CrossRef]
- Iora, S.R.; Maciel, G.M.; Zielinski, A.A.; da Silva, M.V.; Pontes, P.V.d.A.; Haminiuk, C.W.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; dos Santos, L.F.; Franchin, M.; Pereira, G.E.; de Souza Vieira, T.M.F.; Rosalen, P.L.; de Alencar, S.M. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera L. var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol 2022, 343, 126158. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.M. The use and importance of design of experiments (DOE) in process modelling in food science and technology. In Mathematical and Statistical Methods in Food Science and Technology, 1st ed.; Granato, D., Ares, G., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as high-performance green co-solvents in the aqueous extraction of polyphenols and anthocyanin pigments from solid onion waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Makris, D.P.; Psarra, E.; Kallithraka, S.; Kefalas, P. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res. Int. 2003, 36, 805–814. [Google Scholar] [CrossRef]
- Makris, D.; Kefalas, P. Characterization of polyphenolic phytochemicals in red grape pomace. Int. J. Waste Resour 2013, 3, 126. [Google Scholar] [CrossRef]










| CCA (% w/v) | t (min) | CSF | CSF’ | YTP (mg GAE g−1 DM) |
|---|---|---|---|---|
| 5 | 60 | −0.45 a | 6.55 a | 123.06 a |
| 180 | 0.03 c | 7.03 c | 126.13 a | |
| 300 | 0.25 c | 7.25 c | 149.68 c | |
| 10 | 60 | −0.26 a | 6.74 a | 134.52 c |
| 180 | 0.22 c | 7.22 c | 145.05 c | |
| 300 | 0.44 b | 7.44 b | 163.23 b | |
| 15 | 60 | −0.12 c | 6.88 c | 123.73 a |
| 180 | 0.36 c | 7.36 c | 145.09 c | |
| 300 | 0.58 b | 7.58 b | 168.49 b |
| Design Point | Independent Variables | Responses | ||
|---|---|---|---|---|
| YTP (mg GAE g−1 DM) | ||||
| X1 (CCA, %) | X2 (t, min) | Measured | Predicted | |
| 1 | −1 (5) | −1 (60) | 133 | 130 |
| 2 | −1 (5) | 1 (300) | 150 | 147 |
| 3 | 1 (15) | −1 (60) | 124 | 126 |
| 4 | 1 (15) | 1 (300) | 168 | 170 |
| 5 | −1 (5) | 0 (180) | 126 | 132 |
| 6 | 1 (15) | 0 (180) | 146 | 141 |
| 7 | 0 (10) | −1 (60) | 135 | 135 |
| 8 | 0 (10) | 1 (300) | 165 | 166 |
| 9 | 0 (10) | 0 (180) | 145 | 144 |
| 10 | 0 (10) | 0 (180) | 147 | 144 |
| 11 | 0 (10) | 0 (180) | 140 | 144 |
| Compound | Yield (μg g−1 DM) | |||
|---|---|---|---|---|
| Water | 60% EtOH | 75% Gl | Solvent | |
| Non-pigment polyphenols | ||||
| Gallic acid | 9.1 ± 0.1 a | nd | 15 ± 2 b | nd |
| Caftaric acid | 41.9 ± 0.8 a | 29 ± 1 b | 44 ± 2 a, d | 40 ± 2 a, c |
| Catechin | 136 ± 8 a | 69 ± 3 b | 250 ± 10 c | 300 ± 20 d |
| p-Coumarate derivative | 14.6 ± 0.8 a | nd | nd | 16 ± 1 a |
| Ferulate derivative | 44 ± 1 a | 56 ± 2 b | 55 ± 3 b | 59 ± 6 b |
| p-Coumaric acid | 12.5 ± 0.8 a | 12.6 ± 0.5 a | 14.2 ± 0.4 b | 18 ± 2 c |
| Ferulic acid | 18 ± 1 a | 19 ± 2 a | 19.2 ± 0.9 a | 26.8 ± 0.4 b |
| Rutin | 6.4 ± 0.3 a | 16 ± 1 b | 11.4 ± 0.4 c | 16.5 ± 0.9 b |
| Quercetin 3-O-glucuronide | 47 ± 1 a | 99 ± 1 b | 78.9 ± 0.9 c | 83 ± 5 c |
| Kaempferol 3-O-rutinoside | nd | 6.7 ± 0.2 a | 2.29 ± 0.08 b | 3.5 ± 0.4 c |
| Myricetin | 17.5 ± 0.4 a | 92 ± 4 b | 45.0 ± 0.9 c | 50 ± 4 c |
| Resveratrol | 9.4 ± 0.1 a | 21.6 ± 0.8 b | 17.1 ± 0.2 c | 21 ± 1 b |
| Quercetin | 19.2 ± 0.2 a | 171 ± 5 b | 109 ± 4 c | 149 ± 9 d |
| Kaempferol | 7.8 ± 0.4 a | 32 ± 1 b | 18.9 ± 0.3 c | 30.8 ± 0.6 b |
| Isorhamnetin | 9.8 ± 0.1 a | 93 ± 1 b | 46.4 ± 0.4 c | 90 ± 5 b |
| Total | 394 | 718 | 730 | 901 |
| Anthocyanin pigments | ||||
| Cyanidin 3-O-glucoside | 24.2 ± 0.2 a | 27 ± 3 a | 5.3 ± 0.4 b | 13 ± 2 c |
| Delphinidin 3-O-glucoside | 65 ± 6 a | 78 ± 3 b | 61 ± 7 c | 115 ± 2 d |
| Petunidin 3-O-glucoside | 85 ± 5 a | 390 ± 24 b | 97 ± 8 a | 180 ± 10 c |
| Paeonidin 3-O-glucoside | 364 ± 4 a | 730 ± 40 b | 399 ± 6 c | 250 ± 20 d |
| Malvidin 3-O-glucoside | 36 ± 2 a | 131 ± 6 b | 65 ± 2 c | 74 ± 7 c |
| Malvidin 3-O-glucoside p-coumarate | 58 ± 1 a | 1320 ± 30 b | 315 ± 6 c | 420 ± 30 d |
| Total | 631 | 2676 | 942 | 1049 |
| Sum | 1025 | 3394 | 1671 | 1950 |
| Variable | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CCA (%) | X1 | 5 | 10 | 15 |
| t (min) | X2 | 60 | 180 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geropoulou, M.; Yiagtzi, E.; Chatzimitakos, T.; Palaiogiannis, D.; Makris, D.P. Organosolv Treatment of Red Grape Pomace for Effective Recovery of Antioxidant Polyphenols and Pigments Using a Ternary Glycerol/Ethanol/Water System under Mild Acidic Conditions. Molecules 2024, 29, 563. https://doi.org/10.3390/molecules29030563
Geropoulou M, Yiagtzi E, Chatzimitakos T, Palaiogiannis D, Makris DP. Organosolv Treatment of Red Grape Pomace for Effective Recovery of Antioxidant Polyphenols and Pigments Using a Ternary Glycerol/Ethanol/Water System under Mild Acidic Conditions. Molecules. 2024; 29(3):563. https://doi.org/10.3390/molecules29030563
Chicago/Turabian StyleGeropoulou, Maria, Elissavet Yiagtzi, Theodoros Chatzimitakos, Dimitrios Palaiogiannis, and Dimitris P. Makris. 2024. "Organosolv Treatment of Red Grape Pomace for Effective Recovery of Antioxidant Polyphenols and Pigments Using a Ternary Glycerol/Ethanol/Water System under Mild Acidic Conditions" Molecules 29, no. 3: 563. https://doi.org/10.3390/molecules29030563