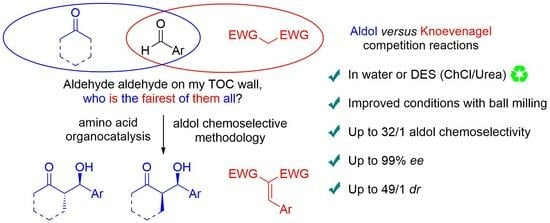
Enantioselective Catalytic Aldol Reactions in the Presence of Knoevenagel Nucleophiles: A Chemoselective Switch Optimized in Deep Eutectic Solvents Using Mechanochemistry
Abstract
:1. Introduction
2. Discussion and Results
3. Materials and Methods
3.1. General Procedure for the Preparation of the Deep Eutectic Solvents (DES)
- ChCl:Urea (1:2)
- ChCl:Glycerol (1:2)
3.2. Typical Competition Experimental Procedure for the Knoevenagel versus Aldol Reaction in DES
3.3. Typical Competition Experimental Procedure for the Knoevenagel versus Aldol Reaction in H2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahrwald, R. Enantioselective Organocatalyzed Reactions; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Berkessel, A.; Gröger, H. Asymmetric Organocatalysis—From Biomimetic Concepts to Applications in Asymmetric Synthesis; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Dalko, P. Enantioselective Organocatalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Juaristi, E. Recent Developments in Next Generation (S)-Proline-Derived Chiral Organocatalysts. Tetrahedron 2021, 88, 132143. [Google Scholar] [CrossRef]
- Krištofíková, D.; Modrocká, V.; Mečiarová, M.; Šebesta, R. Green Asymmetric Organocatalysis. ChemSusChem 2020, 13, 2828–2858. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.G.; Juaristi, E. Recent Efforts Directed to the Development of More Sustainable Asymmetric Organocatalysis. Chem. Commun. 2012, 48, 5396–5409. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Alonso, D.A.; Burlingham, S.-J.; Chinchilla, R.; Guillena, G.; Ramón, D.J.; Tiecco, M. Asymmetric Organocatalysis in Deep Eutectic Solvents. Eur. J. Org. Chem. 2021, 2021, 4065–4071. [Google Scholar] [CrossRef]
- Guajardo, N.; Müller, C.R.; Schrebler, R.; Carlesi, C.; Domínguez de María, P. Deep Eutectic Solvents for Organocatalysis, Biotransformations, and Multistep Organocatalyst/Enzyme Combinations. ChemCatChem 2016, 8, 1020–1027. [Google Scholar] [CrossRef]
- Fanjul-Mosteirín, N.; del Amo, V. Organocatalytic Transformations in Deep Eutectic Solvents: Green Methodologies Made Greener. Tetrahedron 2021, 84, 131967. [Google Scholar] [CrossRef]
- Tietze, L.F.; Beifuss, U. The Knoevenagel Reaction in Comprehensive Organic Synthesis; Trost, B.M., Ed.; Pergamon: Oxford, UK, 1991; Volume 2, pp. 341–394. [Google Scholar]
- van Beurden, K.; de Koning, S.; Molendijk, D.; van Schijndel, J. The Knoevenagel Reaction: A Review of the Unfinished Treasure Map to Forming Carbon–Carbon Bonds. Green Chem. Lett. Rev. 2020, 13, 349–364. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Al Farouk, F.O.; Sherif, S.M.; Karaghiosoff, K. Synthesis of Progesterone Derivatives and Evaluation of Their Efficiency as Pneumococcal Vaccines. Med. Chem. Res. 2014, 23, 3165–3177. [Google Scholar] [CrossRef]
- Tan, H.; Chen, X.; Chen, H.; Liu, H.; Qiu, S. Proline-Catalyzed Knoevenagel Condensation/[4+2] Cycloaddition Cascade Reaction: Application to Formal Synthesis of Averufin. Eur. J. Org. Chem. 2015, 2015, 4956–4963. [Google Scholar] [CrossRef]
- Gomes, J.; Daeppen, C.; Liffert, R.; Roesslein, J.; Kaufmann, E.; Heikinheimo, A.; Neuburger, M.; Gademann, K. Formal Total Synthesis of (−)-Jiadifenolide and Synthetic Studies Toward Seco-Prezizaane-Type Sesquiterpenes. J. Org. Chem. 2016, 81, 11017–11034. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Das, B. Efficient Organocatalyzed Solvent-Free Selective Synthesis of Conjugated Enones. Tetrahedron Lett. 2009, 50, 897–900. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.-H.; Guan, Z. A Simple Method for the Preparation of Functionalized Trisubstituted Alkenes and α,β,γ,δ-Unsaturated Carbonyl Compounds by Using Natural Amino Acid L-Tryptophan. Catal. Commun. 2010, 11, 656–659. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Houk, K.N.; Martin, H.J.; List, B. Quantum Mechanical Predictions of the Stereoselectivities of Proline-Catalyzed Asymmetric Intermolecular Aldol Reactions. J. Am. Chem. Soc. 2003, 125, 2475–2479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liang, Z.; Wu, X.; Lu, Y. Asymmetric Aldol Reactions Catalyzed by Tryptophan in Water. Chem. Commun. 2006, 2801–2803. [Google Scholar] [CrossRef]
- Karmakar, A.; Maji, T.; Wittmann, S.; Reiser, O. L-Proline/CoCl2-Catalyzed Highly Diastereo- and Enantioselective Direct Aldol Reactions. Chem. Eur. J. 2011, 17, 11024–11029. [Google Scholar] [CrossRef]
- Nugent, T.C.; Goswami, F.; Debnath, S.; Hussain, I.; Hussein, H.A.E.D.; Karn, A.; Nakka, S. Harnessing Additional Capability from in Water Reaction Conditions: Aldol versus Knoevenagel Chemoselectivity. Adv. Synth. Catal. 2021, 363, 3539–3545. [Google Scholar] [CrossRef]
- Nugent, T.C.; de Vos, A.E.; Hussein, H.A.E.D.; Goswami, F. A 2000 to 2020 Practitioner’s Guide to Chiral Amine Based Enantioselective Aldol Reactions: Ketone Substrates, Best Methods, in Water Reaction Environments, and Defining Nuances. Eur. J. Org. Chem. 2022, 2022, e202100529. [Google Scholar] [CrossRef]
- Nugent, T.C.; Spiteller, P.; Hussain, I.; Hussein, H.A.E.D.; Najafian, F.T. A Catalyst-Directed Remote Stereogenic Center Switch During the Site-Selective Aldol Desymmetrization of Cyclohexanone-Based Diketones. Adv. Synth. Catal. 2016, 358, 3706–3713. [Google Scholar] [CrossRef]
- Umehara, A.; Kanemitsu, T.; Nagata, K.; Itoh, T. Stereoselective synthesis of vic-halohydrins via L-tert-leucine-catalyzed syn-selective aldol reaction. Synlett 2012, 23, 453–457. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Wang, Y.-Z.; Gong, L.-Z. Design of Organocatalysts for Asymmetric Direct syn-Aldol Reactions. Org. Lett. 2007, 9, 4247–4249. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, N.; Imai, M.; Ramasastry, S.S.V.; Barbas, C.F., III. Mimicking Aldolases through Organocatalysis: syn-Selective Aldol Reactions with Protected Dihydroxyacetone. Org. Lett. 2007, 9, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Kumar, V.; Chimni, S.S. Asymmetric syn-Selective Direct Aldol Reaction of Protected Hydroxyacetone Catalyzed by Primary Amino Acid Derived Bifunctional Organocatalyst in the Presence of Water. Org. Biomol. Chem. 2011, 9, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Hikawa, R.; Shimogaki, M.; Kano, T. Design of Threonine-Derived Amino Sulfonamide Organocatalysts for the Highly Stereoselective Aldol Reactions. Asian J. Org. Chem. 2023, 12, e202300113. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Z.; Shen, H.-M.; Lu, Y. Highly Efficient Threonine-Derived Organocatalysts for Direct Asymmetric Aldol Reactions in Water. Adv. Synth. Catal. 2007, 349, 812–816. [Google Scholar] [CrossRef]
- Rodríguez, B.; Bruckmann, A.; Bolm, C. A Highly Efficient Asymmetric Organocatalytic Aldol Reaction in a Ball Mill. Chem. Eur. J. 2007, 13, 4710–4722. [Google Scholar] [CrossRef]
- Sapir, L.; Harries, D. Restructuring a Deep Eutectic Solvent by Water: The Nanostructure of Hydrated Choline Chloride/Urea. J. Chem. Theory Comput. 2020, 16, 3335–3342. [Google Scholar] [CrossRef]
- Liu, H.; Peng, L.; Zhang, T.; Li, Y. L-Proline Catalyzed Asymmetric Aldol Reactions of Protected Hydroxyacetone. New J. Chem. 2003, 27, 1159–1160. [Google Scholar] [CrossRef]
- Kitanosono, T.; Kobayashi, S. Reactions in Water Involving the “On-Water” Mechanism. Chem. Eur. J. 2020, 26, 9408–9429. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Miura, T.; Imai, K.; Ina, M.; Tada, N.; Imai, N.; Itoh, A. Direct Asymmetric Aldol Reaction with Recyclable Fluorous Organocatalyst. Org. Lett. 2010, 12, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Bai, S.; Gao, Q.; Liu, Y.; Yang, Q. Acid Controlled Diastereoselectivity in Asymmetric Aldol Reaction of Cycloketones with Aldehydes using Enamine-Based Organocatalysts. Chem. Commun. 2011, 47, 6716–6718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Cheng, Q.; Su, Y.; Li, H.; Xiao, R.; Tan, C.; Liu, G. A Chemo-Enzymatic Oxidation/Aldol Sequential Process Directly Converts Arylbenzyl Alcohols and Cyclohexanol into Chiral β-Hydroxy Carbonyls. Green Chem. 2021, 23, 7773–7779. [Google Scholar]
- Mase, N.; Nakai, Y.; Ohara, N.; Yoda, H.; Takabe, K.; Tanaka, F.; Barbas, C.F., III. Organocatalytic Direct Asymmetric Aldol Reactions in Water. J. Am. Chem. Soc. 2006, 128, 734–735. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Liang, J.; Shang, Z. Catalysis by L-Lysine: A Green Method for the Condensation of Aromatic Aldehydes with Acidic Methylene Compounds in Water at Room Temperature. Chin. J. Chem. 2010, 28, 2255–2259. [Google Scholar] [CrossRef]
- Inoue, H.; Kikuchi, M.; Ito, J.-i.; Nishiyama, H. Chiral Phebox-Rhodium Complexes as Catalysts for Asymmetric Direct Aldol Reaction. Tetrahedron 2008, 64, 493–499. [Google Scholar] [CrossRef]
- Massa, A.; Scettri, A.; Filosa, R.; Capozzolo, L. Synthesis of β-Hydroxymalonates: The Direct Aldol Addition of Malonates to Aldehydes in the Presence of SiCl4 and i-Pr2EtN. Tetrahedron Lett. 2009, 50, 7318–7321. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yu, M.; Zhao, G.; Wang, S. Highly Efficient and Reusable Dendritic Catalysts Derived from N-Prolylsulfonamide for the Asymmetric Direct Aldol Reaction in Water. Org. Lett. 2006, 8, 4417–4420. [Google Scholar] [CrossRef]
- Felpin, F.-X.; Miqueu, K.; Sotiropoulos, J.-M.; Fouquet, E.; Ibarguren, O.; Laudien, J. Room-Temperature, Ligand- and Base-Free Heck Reactions of Aryl Diazonium Salts at Low Palladium Loading: Sustainable Preparation of Substituted Stilbene Derivatives. Chem. Eur. J. 2010, 16, 5191–5204. [Google Scholar] [CrossRef]
- He, L.; Tang, Z.; Cun, L.; Mi, A.; Jiang, Y.; Gong, L. L-Proline Amide-Catalyzed Direct Asymmetric Aldol Reaction of Aldehydes with Chloroacetone. Tetrahedron 2006, 62, 346–351. [Google Scholar] [CrossRef]
- Kim, K.-M.; Park, I.-H. A Convenient Halogenation of α, β-Unsaturated Carbonyl Compounds with OXONE® and Hydrohalic acid (HBr, HCl). Synthesis 2004, 16, 2641–2644. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sumiya, T.; Takahashi, J.; Gotoh, H.; Urushima, T.; Shoji, M. Highly diastereo- and enantioselective direct aldol reactions in water. Angew. Chem. Int. Ed. 2006, 45, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Martínez-CastaÇeda, A.; Poladura, B.; Rodríguez-Solla, H.; Concellón, C.; Amo, V.D. Highly Enantioselective Proline-Catalysed Direct Aldol Reaction of Chloroacetone and Aromatic Aldehydes. Chem. Eur. J. 2012, 18, 5188–5190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, C.; Long, X.; Fu, X.; Chen, G.; Liu, Z. Simple Proline Derivatives as Recoverable Catalysts for the Large-Scale Stoichiometric Aldol Reactions. Catal. Sci. Technol. 2012, 2, 1068–1071. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, X.; Wang, Y. A Green and Efficient Asymmetric Aldol Reaction Catalyzed by a Chiral Anion Modified Ionic Liquid. Eur. J. Org. Chem. 2010, 3672–3677. [Google Scholar] [CrossRef]
- Giacalone, F.; Gruttadauria, M.; Meo, P.L.; Riela, S.; Noto, R. New Simple Hydrophobic Proline Derivatives as Highly Active and Stereoselective Catalysts for the Direct Asymmetric Aldol Reaction in Aqueous Medium. Adv. Synth. Catal. 2008, 350, 2747–2760. [Google Scholar] [CrossRef]
- Yadav, G.D.; Singh, S. (l)-Prolinamide Imidazolium Hexafluorophosphate Ionic Liquid as an Efficient Reusable Organocatalyst for Direct Asymmetric Aldol Reaction in Solvent-Free Condition. RCS Adv. 2016, 6, 100459–100466. [Google Scholar] [CrossRef]
- Patterson, J.W.; Nelson, J.T. A Hantzsch 1,4-Dihydropyridine Synthesis Producing an Unusual By-Product. J. Heterocyclic Chem. 1988, 25, 125–128. [Google Scholar] [CrossRef]
- Ramasastry, S.S.V.; Albertshofer, K.; Utsumi, N.; Tanaka, F.; Barbas, C.F., III. Mimicking Fructose and Rhamnulose Aldolases: Organocatalytic syn-Aldol Reactions with Unprotected Dihydroxyacetone. Angew. Chem. Int. Ed. 2007, 46, 5572–5575. [Google Scholar] [CrossRef]
- Wang, R.; Xu, E.; Su, Z.; Duan, H.; Wang, J.; Xue, L.; Lin, Y.; Li, Y.; Weia, Z.; Yang, Q. Preparation of Prolinamide with Adamantane for Aldol Reaction Catalysis in Brine and Separation using a Poly(AN-MA-β-CD) Nanofibrous Film via Host–Guest Interaction. RSC Adv. 2018, 8, 28376–28385. [Google Scholar] [CrossRef] [PubMed]




| Entry | H2O b (equiv) | Chemoselectivity 4/5 c | Yield(%) d (anti-&syn-4) | dr (anti-/syn-4) | ee (anti-4) |
|---|---|---|---|---|---|
| 1 | 0 | 8:1 | 78 | 5:1 | 93 |
| 2 | 3.0 | 14:1 | 83 | >19:1 | 98 |
| 3 | 7.5 | 14:1 | 78 | >19:1 | 99 |
| 4 | 15.0 | 13:1 | 84 | >19:1 | 98 |
| 5 | 30.0 | 15:1 | 79 | >19:1 | 98 |
| 6 | 15.0 e | 13:1 | 87 | 13:1 | 98 |
| 7 f | 15.0 | 7:1 | 78 | 14:1 | 98 |
| Entry | Knoevenagel Nucleophile | Major Competition Product (anti-4) | Aldol (4)/ Knoevenagel (5) Chemoselectivity b | Time (h) | Yield(%) (anti-4) c | dr (4) d | ee(anti-4) |
|---|---|---|---|---|---|---|---|
| 1 |  |  | 13:1 | 40 | 84 e | >19:1 | 98 |
| 2 |  |  | 18:1 | 36 | 90 e | >19:1 | 99 |
| 3 |  |  | 8:1 | 40 | 73 | >19:1 | 99 |
| 4 |  |  | 17:1 | 36 | 83 | >19:1 | 99 |
| 5 f |  |  | >19:1 | 20 | 61 | >19:1 | 99 |
| 6 f |  |  | >19:1 | 24 | 83 | 17:1 | 99 |
| 7 |  |  | 15:1 | 30 | 82 | 17:1 | 98 |
| 8 f |  |  | 11:1 | 42 | 59 | 10:1 | 95 |
| 9 f |  |  | >19:1 | 36 | 82 | 14:1 | 98 |
| 10 f |  |  | 10:1 | 28 | 65 | 13:1 17:1 g | 98 |
| 11 |  |  | >19:1 | 24 | 94 e | 4:1 | 99 |
| Entry | Knoevenagel Nucleophile | Major Competition Product (syn-4) | Aldol (4)/ Knoevenagel (5) Chemoselectivity b | Time (h) | Yield(%) c | dr (4) d | ee (syn-4) |
|---|---|---|---|---|---|---|---|
| 1 e |  |  | 7:1 | 30 | 63 f | 17:1 g | 95 h |
| 2 i |  |  | 16:1 | 30 | 80 | 9:1 | 97 j |
| 3 |  |  | 8:1 | 40 | 77 | 8:1 | 95 j |
| 4 |  |  | >19:1 | 60 | 37 | 8:1 | - k |
 | |||||
|---|---|---|---|---|---|
| Entry | 1 (mol%)/H2O (μL) | Aldol (4)/Knoevenagel (5) Chemoselectivity b | Conversion(%) b | dr (anti/syn-4) b | ee (anti-4) c |
| 1 | 30/0 | 8:1 | 92 | 1.7:1 | 32 d |
| 2 | 30/0 | 7.8:1 | 90 | 5:1 | 93 |
| 3 | 30/200 e | 15:1 | 93 | 8:1 | 96 |
| 4 | 5/200 e | 13:1 | 94 | 9:1 | 99 |
| 5 | 5/0 | 3:1 | 98 | 3.2:1 | 92 |
| 6 | 2.5/200 e | 16:1 | 87 | 8:1 | 93 |
| 7 | 1/200 e | 11:1 | 70 | 2:1 | 93 |
| 8 | 5/100 f | 15:1 | 95 | 7.5:1 | 95 |
| 9 | 5/50 g | 14:1 | 91 | 4.5:1 | 92 |
| 10 | 5/600 h | 12:1 | 95 | 7:1 | 93 |
| Entry | Knoevenagel Nucleophile | Major Competition Product (4) | Aldol (4)/Knoevenagel (5) Chemoselectivity b | Yield (4, %) c | dr (4) b,d | ee (4) e |
|---|---|---|---|---|---|---|
| 1 |  |  | 13:1 | 73 | 9:1 | 99 |
| 2 |  |  | 6:1 | 75 | 8.5:1.5 | 98 |
| 3 |  |  | 10:1 | 70 | 6.5:3.5 | 74 f |
| 4 |  |  | 6:1 | 30 | 9:1 | 98 |
| 5 |  |  | 6:1 | 65 | 9.8:0.2 | 98 g |
| 6 |  |  | 32:1 | 64 | 9.3:0.7 | 96 |
| 7 |  |  | 8:1 | 62 | 9:1 | 99 h |
| 8 |  |  | 8:1 | 60 | 8.8:1.2 9:1 i | 94 |
| 9 |  |  | 6:1 | 58 | 6:4 | 53 j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Beiruty, H.; Zhylinska, S.-S.; Kutateladze, N.; Cheong, H.K.T.; Ñíguez, J.A.; Burlingham, S.J.; Marset, X.; Guillena, G.; Chinchilla, R.; Alonso, D.A.; et al. Enantioselective Catalytic Aldol Reactions in the Presence of Knoevenagel Nucleophiles: A Chemoselective Switch Optimized in Deep Eutectic Solvents Using Mechanochemistry. Molecules 2024, 29, 4. https://doi.org/10.3390/molecules29010004
Al Beiruty H, Zhylinska S-S, Kutateladze N, Cheong HKT, Ñíguez JA, Burlingham SJ, Marset X, Guillena G, Chinchilla R, Alonso DA, et al. Enantioselective Catalytic Aldol Reactions in the Presence of Knoevenagel Nucleophiles: A Chemoselective Switch Optimized in Deep Eutectic Solvents Using Mechanochemistry. Molecules. 2024; 29(1):4. https://doi.org/10.3390/molecules29010004
Chicago/Turabian StyleAl Beiruty, Hanaa, Sofiia-Stefaniia Zhylinska, Nino Kutateladze, Hayley Kay Tinn Cheong, José A. Ñíguez, Sarah J. Burlingham, Xavier Marset, Gabriela Guillena, Rafael Chinchilla, Diego A. Alonso, and et al. 2024. "Enantioselective Catalytic Aldol Reactions in the Presence of Knoevenagel Nucleophiles: A Chemoselective Switch Optimized in Deep Eutectic Solvents Using Mechanochemistry" Molecules 29, no. 1: 4. https://doi.org/10.3390/molecules29010004