Myo-Inositol, Scyllo-Inositol, and Other Minor Carbohydrates as Authenticity Markers for the Control of Italian Bulk, Concentrate, and Rectified Grape Must
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inter-Laboratory Comparison and Method Validation
2.2. Influence of Concentration on the Polyalcohol Content
2.3. Content of Polyols and Minor Carbohydrates in Grape Musts
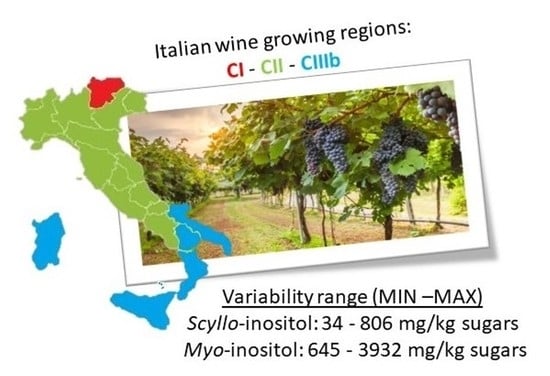
2.4. Seasonal and Geographical Effects on the Variability of Polyols and Minor Carbohydrates
2.5. Identification of Lower Limits for Myo- and Scyllo-Inositol and Higher Limits for Other Minor Sugars
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sampling
3.3. Sugar Derivatization and GC Analysis
3.3.1. Lab#1 Analytical Method
3.3.2. Lab#2 Analytical Method
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Organisation of Vine and Wine (OIV). International Standard for the Labelling of Wines; OIV: Paris, France, 2015.
- Thang, V.H.; Novalin, S. Production of Rectified Grape Musts by Ion Exclusion Chromatography. J. Food Eng. 2006, 76, 128–138. [Google Scholar] [CrossRef]
- Deiana, A.C.; Granados, D.L.; Petkovic, L.M.; Sardella, M.F.; Silva, H.S. Use of Grape Must as a Binder to Obtain Activated Carbon Briquettes. Braz. J. Chem. Eng. 2004, 21, 585–591. [Google Scholar] [CrossRef]
- Pompei, C.; Rossi, M.; Barozzi, E. Rectified Concentrated Grape Must: HPLC Analysis of Phenolic Substances and Hydroxymethylfurfural. J. Food Sci. 1986, 51, 1498–1500. [Google Scholar] [CrossRef]
- Johnson, H.; Robinson, J. The World Atlas of Wine, 8th ed.; Mitchell Beazley: London, UK, 2019; ISBN 9781784726188. [Google Scholar]
- Holmberg, L. Wine Fraud. Int. J. Wine Res. 2010, 2010, 105–113. [Google Scholar] [CrossRef]
- Commission Regulation (EEC) No 2676/90 of 17 September 1990 Determining Community Methods for the Analysis of Wines. European Commission; Official Journal of the European Communities. 1990. Available online: http://data.europa.eu/eli/reg/1990/2676/oj (accessed on 17 April 2023).
- O’Leary, M.H. Carbon Isotopes in Photosynthesis. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Christoph, N.; Rossmann, A.; Schlicht, C.; Voerkelius, S. Wine Authentication Using Stable Isotope Ratio Analysis: Significance of Geographic Origin, Climate, and Viticultural Parameters. ACS Symp. Ser. 2006, 952, 166–179. [Google Scholar]
- Sanz, M. Inositols and Carbohydrates in Different Fresh Fruit Juices. Food Chem. 2004, 87, 325–328. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Darnell, B. Myo-Inositol Content of Common Foods: Development of a High-Myo-Inositol Diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- de Smedt, P.; Liddle, P.A.P.; Cresto, B.; Bossard, A. The Analysis of Non-Volatile Constituents of Wine By Glass Capillary Gas Chromatography. J. Inst. Brew. 1981, 87, 349–351. [Google Scholar] [CrossRef]
- Villamiel, M.; del Castillo, M.D.; Martín, C.S.; Corzo, N. Assessment of the Thermal Treatment of Orange Juice during Continuous Microwave and Conventional Heating. J. Sci. Food Agric. 1998, 78, 196–200. [Google Scholar] [CrossRef]
- Monetti, A.; Versini, G.; Dalpiaz, G.; Reniero, F. Sugar Adulterations Control in Concentrated Rectified Grape Musts by Finite Mixture Distribution Analysis of the Myo- and Scyllo-Inositol Content and the D/H Methyl Ratio of Fermentative Ethanol. J. Agric. Food Chem. 1996, 44, 2194–2201. [Google Scholar] [CrossRef]
- Regulation (EU) No.1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No.922/72, (EEC) No.234/79, (EC) No.1037/2001 and (EC) No.1234/2007. European Commission; European Competition Law. 2013. Available online: http://data.europa.eu/eli/reg/2013/1308/oj (accessed on 17 April 2023).
- Versini, G.; Dalla, S.A.; Margheri, G. Polyalcohols and secondary sugars in rectified concentrated musts. Possible parameters of genuineness? Vignevini 1984. (In Italian) [Google Scholar]
- Resolution OIV-OENO 419C-2015: Specific Methods for the Analysis of Grape Sugar (Rectified Concentrated Must)—Determination of Meso-Inositol, Scyllo-Inositol and Sucrose; OIV: Paris, France, 2015.
- 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C(2002) 3044). European Commission; Official Journal of the European Communities. 2002. Available online: http://data.europa.eu/eli/dec/2002/657/oj (accessed on 17 April 2023).
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized Guidelines for Single-Laboratory Validation of Methods of Analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Rectified Concentrated Grape Must: Report. Available online: https://paperpile.com/app/p/3dbd55f4-043c-04b7-8e10-91fc563372d9 (accessed on 23 March 2022).
- Perini, M.; Nardin, T.; Camin, F.; Malacarne, M.; Larcher, R. Combination of Sugar and Stable Isotopes Analyses to Detect the Use of Nongrape Sugars in Balsamic Vinegar Must. J. Mass Spectrom. 2018, 53, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Loewus, M.W. Myo-Inositol: Its Biosynthesis and Metabolism. Annu. Rev. Plant Physiol. 1983, 34, 137–161. [Google Scholar] [CrossRef]
- Siracusa, L.; Napoli, E.; Ruberto, G. Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules 2022, 27, 1525. [Google Scholar] [CrossRef] [PubMed]
- Society for Experimental Biology (Great Britain). Storage Carbohydrates in Vascular Plants: Distribution, Physiology and Metabolism; CUP Archive; Cambridge University Press: Cambridge, UK, 1984; ISBN 9780521236980. [Google Scholar]
- Brunetti, M.; Buffoni, L.; Maugeri, M.; Nanni, T. Trends of Minimum and Maximum Daily Temperatures in Italy from 1865 to 1996. Theor. Appl. Climatol. 2000, 66, 49–60. [Google Scholar] [CrossRef]
- Bock, A.; Sparks, T.H.; Estrella, N.; Menzel, A. Climate-Induced Changes in Grapevine Yield and Must Sugar Content in Franconia (Germany) between 1805 and 2010. PLoS ONE 2013, 8, e69015. [Google Scholar] [CrossRef] [PubMed]
- Torquati, B.; Giacchè, G.; Venanzi, S. Economic Analysis of the Traditional Cultural Vineyard Landscapes in Italy. J. Rural. Stud. 2015, 39, 122–132. [Google Scholar] [CrossRef]
- Silva, B.M.; Seabra, R.M.; Andrade, P.B.; Oliveira, M.B.; Ferreira, M.A. Adulteração Por Adição De Açúcares A Sumos De Frutos: Uma Revisão Adulteration of Fruit Juice By Addition of Sugars: A Review Adulteración Por Adición De Azúcares A Zumos De Frutas: Una Revisión. Cienc. Y Tecnol. Aliment. 1999, 2, 184–191. [Google Scholar] [CrossRef]
- Lees, J.-F.M. FoodIntegrity Handbook—A Guide to Food Authenticity Issues and Analytical Solutions; Eurofins Analytics France: Paris, France, 2018. [Google Scholar]





| Compound | Correlation Coefficient (R2) | Slope ± STD | LOD (mg/L Grape Must) | LOQ (mg/L Grape Must) | Recovery (%) Low Level | Recovery (%) High Level | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab#1 | Lab#2 | Lab#1 | Lab#2 | Lab#1 | Lab#2 | Lab#1 | Lab#2 | Lab#1 | Lab#2 | Lab#1 | Lab#2 | |
| sorbitol | 0.997 | 0.998 | 0.0039 ± 0.0005 | 2.04 ± 0.20 | 0.7 | 0.2 | 2.0 | 0.5 | 95 | 104 | 97 | 104 |
| scyllo-inositol | 0.998 | 0.998 | 0.0058 ± 0.0004 | 4.07 ± 0.25 | 0.3 | 0.2 | 1.0 | 0.5 | 99 | 112 | 105 | 112 |
| myo-inositol | 0.999 | 0.997 | 0.0053 ± 0.0003 | 3.92 ± 0.44 | 0.3 | 0.2 | 1.0 | 0.5 | 105 | 100 | 96 | 100 |
| sucrose | 0.997 | 0.995 | 0.0014 ± 0.0009 | 2.10 ± 0.81 | 0.7 | 0.2 | 2.0 | 0.5 | 102 | 99 | 104 | 99 |
| lactose | 0.996 | 0.997 | 0.0025 ± 0.0011 | 2.85 ± 0.73 | 0.7 | 0.2 | 2.0 | 0.5 | 97 | 99 | 101 | 99 |
| maltose | 0.998 | 0.996 | 0.0022 ± 0.0008 | 3.56 ± 0.83 | 0.7 | 0.2 | 2.0 | 0.5 | 95 | 98 | 95 | 98 |
| isomaltose | 0.997 | 0.997 | 0.0022 ± 0.0009 | 3.07 ± 0.87 | 0.7 | 0.2 | 2.0 | 0.5 | 106 | 100 | 98 | 100 |
| Natural Grape Must | CM | |||||||
|---|---|---|---|---|---|---|---|---|
| Brix | Scyllo-Inositol | Myo-Inositol | Myo/Scyllo | Brix | Scyllo-Inositol | Myo-Inositol | Myo/Scyllo | |
| (mg/kg Sugar) (SD) | (mg/kg Sugar) (SD) | (mg/kg Sugar) (SD) | (mg/kg Sugar) (SD) | |||||
| GM 1 | 6.9 | 129 (11) | 1364 (50) | 11 | 69.2 | 112 (5) | 1302 (15) | 12 |
| GM 2 | 5.9 | 104 (8) | 1829 (70) | 18 | 70.1 | 115 (3) | 1929 (80) | 17 |
| GM 3 | 5.3 | 106 (7) | 1322 (30) | 12 | 67.7 | 111 (8) | 1309 (69) | 12 |
| Concentration (mg/kg Sugar) | Median | SD | Q1 | Q2 | |||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | |||||
| Scyllo-inositol | 161 | 34 | 806 | 136 | 98 | 97 | 199 |
| Myo-inositol | 1472 | 645 | 3932 | 1346 | 565 | 1055 | 1764 |
| Sorbitol | 120 | <0.7 | 1207 | 77 | 144 | 26 | 156 |
| Sucrose | 65 | <0.7 | 534 | 37 | 82 | 11 | 94 |
| Lactose | 130 | 21 | 390 | 116 | 69 | 79 | 169 |
| Maltose | 232 | <0.7 | 2222 | 107 | 332 | 22 | 299 |
| Isomaltose | 89 | <0.7 | 1639 | 42 | 156 | 5 | 110 |
| Italian Wine-Growing Regions | Scyllo-Inositol | Myo-Inositol | Myo-Inositol /Scyllo-Inositol Mean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg Sugar) Min–Max | Mean | Median | SD | Q1 | Q2 | (mg/kg Sugar) Min–Max | Mean | Median | SD | Q1 | Q2 | ||
| CI | 34–122 | 73 | 71 | 23 | 57 | 89 | 645–1372 | 1000 | 1023 | 219 | 792 | 1160 | 15 |
| CII | 34–485 | 143 | 127 | 43 | 97 | 166 | 759–3830 | 1437 | 1321 | 218 | 1024 | 1685 | 12 |
| CIIIb | 60–806 | 230 | 203 | 108 | 139 | 278 | 756–3932 | 1700 | 1617 | 732 | 1177 | 2132 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolini, M.; Perini, M.; Allari, L.; Tonidandel, L.; Finato, F.; Guardini, K.; Larcher, R. Myo-Inositol, Scyllo-Inositol, and Other Minor Carbohydrates as Authenticity Markers for the Control of Italian Bulk, Concentrate, and Rectified Grape Must. Molecules 2023, 28, 3609. https://doi.org/10.3390/molecules28083609
Paolini M, Perini M, Allari L, Tonidandel L, Finato F, Guardini K, Larcher R. Myo-Inositol, Scyllo-Inositol, and Other Minor Carbohydrates as Authenticity Markers for the Control of Italian Bulk, Concentrate, and Rectified Grape Must. Molecules. 2023; 28(8):3609. https://doi.org/10.3390/molecules28083609
Chicago/Turabian StylePaolini, Mauro, Matteo Perini, Letizia Allari, Loris Tonidandel, Fabio Finato, Katia Guardini, and Roberto Larcher. 2023. "Myo-Inositol, Scyllo-Inositol, and Other Minor Carbohydrates as Authenticity Markers for the Control of Italian Bulk, Concentrate, and Rectified Grape Must" Molecules 28, no. 8: 3609. https://doi.org/10.3390/molecules28083609