Vibrational Study on the Structure, Bioactivity, and Silver Adsorption of Silk Fibroin Fibers Grafted with Methacrylonitrile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Untreated Silk Fibroin Fibers
2.1.1. Raman Spectra
2.1.2. IR Spectra
2.2. Methacrylonitrile-Grafted Silk Fibroin Fibers
2.2.1. Raman Spectra
2.2.2. IR Spectra
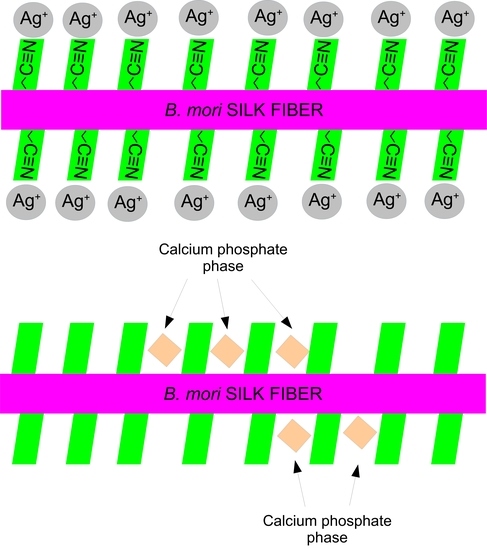
2.3. Silver Adsorption Tests
2.4. Bioactivity Tests in SBF
2.4.1. Raman Spectra
2.4.2. IR Spectra
3. Materials and Methods
3.1. Materials
3.2. Silver Adsorption Tests
3.3. Bioactivity Tests in SBF
3.4. Vibrational Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Adi, D.S.; Ismadi; Damayanti, R.; Subiyanto, B.; Fatriasari, W.; Fudholi, A. A review on natural fibers for development of eco-friendly bio-composite: Characteristics, and utilizations. J. Mater. Res. Technol. 2021, 13, 2442–2458. [Google Scholar] [CrossRef]
- Bayraktar, E.K.; Hockenberger, A.Ş. Investigating the knot performance of silk, polyamide, polyester, and polypropylene sutures. Text. Res. J. 2001, 71, 435–440. [Google Scholar] [CrossRef]
- Zhang, R.; Yahong, H.; Weijie, X.; Liu, F.; Chen, S. Advances in protein-based nanocarriers of bioactive compounds: From microscopic molecular principles to macroscopical structural and functional attributes. J. Agric. Food Chem. 2022, 70, 6354–6367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Atta-ul-Mubeen Shah, S.; Basharat, K.; Qamar, S.A.; Raza, A.; Mohamed, A.; Bilal, M.; Iqbal, H.M.N. Silk-based nano-hydrogels for futuristic biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 72, 103385. [Google Scholar] [CrossRef]
- Griffanti, G.; James-Bashin, M.; Donelli, I.; Freddi, G.; Nazhat, S.N. Functionalization of silk fibroin through anionic fibroin derived polypeptides. Biomed. Mater. 2018, 14, 015006. [Google Scholar] [CrossRef]
- Niu, Q.; Wei, H.; Hsiao, B.S.; Zhang, Y. Biodegradable silk fibroin-based bio-piezoelectric/triboelectric nanogenerators as self-powered electronic devices. Nano Energy 2022, 96, 107101. [Google Scholar] [CrossRef]
- Mao, S.; Sun, B.; Zhou, G.; Guo, T.; Wang, J.; Zhao, Y. Applications of biomemristors in next generation wearable electronics. Nanoscale Horiz. 2022, 7, 822–848. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthcare Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- Janani, G.; Kumar, M.; Chouhan, D.; Moses, J.C.; Gangrade, A.; Bhattacharjee, S.; Mandal, B.B. Insight into Silk-Based Biomaterials: From Physicochemical Attributes to Recent Biomedical Applications. ACS Appl. Bio Mater. 2019, 2, 5460–5491. [Google Scholar] [CrossRef]
- Li, G.; Sun, S. Silk Fibroin-Based Biomaterials for Tissue Engineering Applications. Molecules 2022, 27, 2757. [Google Scholar] [CrossRef]
- Shen, X.; Shi, H.; Wei, H.; Wu, B.; Xia, Q.; Yeo, J.; Huang, W. Engineering natural and recombinant silks for sustainable biodevices. Front. Chem. 2022, 10, 881028. [Google Scholar] [CrossRef]
- Jiao, Z.Y.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.Z.; Chang, P.P.; Kundu, S.C.; Wang, G.B.; Wang, Z.; et al. In Vivo Characterizations of the Immune Properties of Sericin: An Ancient Material with Emerging Value in Biomedical Applications. Macromol. Biosci. 2017, 17, 1700229. [Google Scholar] [CrossRef]
- Kulkarni, G.; Ray, P.G.; Byram, P.K.; Kaushal, M.; Dhara, S.; Das, S. Tailorable hydrogel of gelatin with silk fibroin and its activation/cross-linking for enhanced proliferation of fibroblast cells. Int. J. Biol. Macromol. 2020, 164, 4073–4083. [Google Scholar] [CrossRef]
- Cai, J.Y.; Zhang, L.; Chen, J.; Chen, S.Y. Silk fibroin coating through EDC/NHS cross-link is an effective method to promote graft remodeling of a polyethylene terephthalate artificial ligament. J. Biomater. Appl. 2019, 33, 1407–1414. [Google Scholar] [CrossRef]
- McGill, M.; Coburn, J.M.; Partlow, B.P.; Mu, X.; Kaplan, D.L. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 2017, 63, 76–84. [Google Scholar] [CrossRef]
- Freddi, G.; Massafra, F.R.; Beretta, S.; Shibata, S.; Gotoh, Y.; Yasui, H.; Tsukada, M. Structure and properties of Bombyx mori silk fibers graft with methacrylamide (MAA) and 2-hydroxyethyl methacrylate (HEMA). J. Appl. Polym. Sci. 1996, 60, 1867–1876. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Ishiguro, Y.; Shiozaki, H. Structural analysis of methacrylamide-grafted silk fibers. J. Appl. Polym. Sci. 1993, 50, 1519–1527. [Google Scholar] [CrossRef]
- Tsukada, M.; Gotoh, Y.; Freddi, G.; Yamamoto, T.; Nakabayashi, N. Molecular weight distribution of the methyl methacrylate (MMA) polymer separated from the MMA-grafted silk fiber. J. Appl. Polym. Sci. 1992, 44, 2197–2202. [Google Scholar] [CrossRef]
- Pavoni, E.; Tozzi, S.; Tsukada, M.; Taddei, P. Structural study on methacrylamide-grafted Tussah silk fibroin fibers. Int. J. Biol. Macromol. 2016, 88, 196–205. [Google Scholar] [CrossRef]
- Pavoni, E.; Tsukada, M.; Taddei, P. Influence of grafting with acrylate compounds on the conformational rearrangements of silk fibroin upon electrospinning and treatment with aqueous methanol. J. Raman Spectrosc. 2016, 47, 1367–1374. [Google Scholar] [CrossRef]
- Taddei, P.; Di Foggia, M.; Martinotti, S.; Ranzato, E.; Carmagnola, I.; Chiono, V.; Tsukada, M. Silk fibers grafted with 2-hydroxyethyl methacrylate (HEMA) and 4-hydroxybutyl acrylate (HBA) for biomedical applications. Int. J. Biol. Macromol. 2018, 107, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Taddei, P.; Pavoni, E.; Tsukada, M. Stability toward alkaline hydrolysis of B. mori silk fibroin grafted with methacrylamide. J. Raman Spectrosc. 2016, 47, 731–739. [Google Scholar] [CrossRef]
- Tsukada, M.; Shiozaki, H. Characterization of methacrylonitrile-grafted silk fibers. J. Appl. Polym. Sci. 1990, 39, 1289–1297. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Shiozaki, H.; Pusch, N. Changes in physical properties of methacrylonitrile (MAN)-grafted silk fibers. J. Appl. Polym. Sci. 1993, 49, 593–598. [Google Scholar] [CrossRef]
- Attard, J.; Milescu, R.; Budarin, V.; Matharu, A.S.; Clark, J.H. Unexpected nitrile formation in bio-based mesoporous materials (Starbons®). Chem. Commun. 2018, 54, 686–688. [Google Scholar] [CrossRef]
- Kang, M.; Jung, R.; Kim, H.S.; Youk, J.H.; Jin, H.J. Silver nanoparticles incorporated electrospun silk fibers. J. Nanosci. Nanotechnol. 2007, 7, 3888–3891. [Google Scholar] [CrossRef]
- Zhou, W.H.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.D.; Yu, S.; Zheng, Y.F. Biomimetic AgNPs-antimicrobial peptide/silk fibroin coating for infection-trigger antibacterial capability and enhanced osseointegration. Bioact. Mater. 2023, 20, 64–80. [Google Scholar] [CrossRef]
- Khan, R.S.; Rather, A.H.; Wani, T.U.; Rather, S.U.; Abdal-hay, A.; Sheikh, F.A. A comparative review on silk fibroin nanofibers encasing the silver nanoparticles as antimicrobial agents for wound healing applications. Mater. Today Commun. 2022, 32, 103914. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Colonna, G.M.; Scotti, E.; Boschi, A.; Murakami, R.; Tsukada, M. Absorption of metal cations by modified B. mori silk and preparation of fabrics with anti-microbial activity. J. Appl. Polym. Sci. 2001, 80, 297–303. [Google Scholar] [CrossRef]
- Di Foggia, M.; Tsukada, M.; Taddei, P. Vibrational Study on Structure and Bioactivity of Protein Fibers Grafted with Phosphorylated Methacrylates. Molecules 2021, 26, 6487. [Google Scholar] [CrossRef]
- Taddei, P.; Tsukada, M.; Freddi, G. Affinity of protein fibers towards sulfation. J. Raman Spectrosc. 2013, 44, 190–197. [Google Scholar] [CrossRef]
- Zarembowitch, J.; Maleki, R. The infrared and Raman spectra of methacrylonitrile adducts with copper(I) halides. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1983, 39, 47–51. [Google Scholar] [CrossRef]
- Gribov, L.A.; Demukhamedova, S.D.; Zubkova, O.B. Theoretical analysis and interpretation of vibrational spectra of polyacrylonitrile and polymethacrylonitrile. J. Appl. Spectrosc. 1983, 38, 441–448. [Google Scholar] [CrossRef]
- Panicker, C.Y.; Varghese, H.T.; Harikumar, B.; Chandran, A. Vibrational Spectroscopic Investigations of Methacrylonitrile. Mat. Sci. Res. India 2011, 8, 297–300. [Google Scholar] [CrossRef]
- Deniau, G.; Viel, P.; Bureau, C.; Zalczer, G.; Lixon, P.; Palacin, S. Study of the polymers obtained by electroreduction of methacrylonitrile. J. Electroanal. Chem. 2001, 505, 33–43. [Google Scholar] [CrossRef]
- Wallasch, F.; Abele, M.; Gubler, L.; Wokaun, A.; Müller, K.; Scherer, G.G. Characterization of Radiation-Grafted Polymer Films Using CP/MAS NMR Spectroscopy and Confocal Raman Microscopy. J. Appl. Polym. Sci. 2012, 125, 3500–3508. [Google Scholar] [CrossRef]
- Sun, Y.; Shao, Z.; Hu, P.; Yu, T. Hydrogen bonds in silk fibroin-poly(acrylonitrile-co-methyl acrylate) blends: FT–IR study. J. Polym. Sci. B Polym. Phys. 1997, 35, 1405–1414. [Google Scholar] [CrossRef]
- Alia, J.M.; Edwards, H.G.M. Raman spectroscopic study of the association between acrylonitrile and nickel(II) in aqueous solution; estimation of the enthalpy of association. J. Mol. Struct. 1995, 354, 97–107. [Google Scholar] [CrossRef]
- Ameur, S.; Bureau, C.; Charlier, J.; Palacin, S. Immobilization of Biomolecules on Electrodes Modified by Electrografted Films. J. Phys. Chem. B 2004, 108, 13042–13046. [Google Scholar] [CrossRef]
- Guan, J.P.; Chen, G.Q. Flame resistant modification of silk fabric with vinyl phosphate. Fibers Polym. 2008, 9, 438–443. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Mahdavinia, G.R. Modified chitosan, 7. Graft copolymerization of methacrylonitrile onto chitosan using ammonium persulfate initiator. E-Polymers 2004, 4, 53. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, N.V.; Nadiger, G.S. Crystallinity in silk fibers: Partial acid hydrolysis and related studies. J. Appl. Polym. Sci. 1980, 25, 921–932. [Google Scholar] [CrossRef]
- Saunier, J.; Alloin, F.; Sanchez, J.Y. Electrochemical and spectroscopic studies of polymethacrylonitrile based electrolytes. Electrochem. Acta 2000, 45, 1255–1263. [Google Scholar] [CrossRef]
- Alia, J.M.; Edwards, H.G.M.; Moore, J. Solvation of Ag+ ions in some nitriles; a Fourier transform Raman spectroscopic study. Spectrochim. Acta Part A 1995, 51, 2039–2056. [Google Scholar] [CrossRef]
- Taddei, P.; Monti, P.; Freddi, G.; Arai, T.; Tsukada, M. IR study on the binding mode of metal cations to chemically modified Bombyx mori and Tussah silk fibers. J. Mol. Struct. 2003, 651–653, 433–441. [Google Scholar] [CrossRef]
- Taddei, P.; Arai, T.; Boschi, A.; Monti, P.; Tsukada, M.; Freddi, G. In Vitro Study of the Proteolytic Degradation of Antheraea pernyi Silk Fibroin. Biomacromolecules 2006, 7, 259–267. [Google Scholar] [CrossRef]
- Furuzono, T.; Taguchi, T.; Kishida, A.; Akashi, M.; Tamada, Y. Preparation and characterization of apatite deposited on silk fabric using an alternate soaking process. J. Biomed. Mater. Res. 2000, 50, 344–352. [Google Scholar] [CrossRef]
- Di Foggia, M.; Prati, C.; Gandolfi, M.G.; Taddei, P. An in vitro study on dentin demineralisation and remineralisation: Collagen rearrangements and influence on the enucleated phase. J. Inorg. Biochem. 2019, 193, 84–93. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]



















| Sample | MAN Content w/w% from I2237/IAmide I | MAN Content w/w% from I703/IAmide I | ||||
|---|---|---|---|---|---|---|
| t = 0 | t = 7 days | t = 28 days | t = 0 | t = 7 days | t = 28 days | |
| MAN 10% | 10.3 ± 0.9 | 8.1 ± 0.7 | 8.9 ± 1.2 | 7.8 ± 0.3 | ||
| MAN 25% | 25.6 ± 2.4 | 23.7 ± 0.9 | 24.7 ± 2.5 | 24.0 ± 1.4 | ||
| MAN 32.5% | 32.6 ± 1.0 | 28.4 ± 0.9 | 30.4 ± 1.0 | 28.9 ± 1.2 | ||
| MAN 40% | 39.4 ± 4.1 | 39.1 ± 1.3 | 34.2 ± 2.4 | 39.5 ± 0.6 | 38.4 ± 3.6 | 33.8 ± 1.1 |
| MAN 60% | 60.1 ± 2.3 | 57.3 ± 0.8 | 52.6 ± 1.9 | 61.8 ± 2.4 | 60.4 ± 2.2 | 54.3 ± 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Foggia, M.; Tsukada, M.; Taddei, P. Vibrational Study on the Structure, Bioactivity, and Silver Adsorption of Silk Fibroin Fibers Grafted with Methacrylonitrile. Molecules 2023, 28, 2551. https://doi.org/10.3390/molecules28062551
Di Foggia M, Tsukada M, Taddei P. Vibrational Study on the Structure, Bioactivity, and Silver Adsorption of Silk Fibroin Fibers Grafted with Methacrylonitrile. Molecules. 2023; 28(6):2551. https://doi.org/10.3390/molecules28062551
Chicago/Turabian StyleDi Foggia, Michele, Masuhiro Tsukada, and Paola Taddei. 2023. "Vibrational Study on the Structure, Bioactivity, and Silver Adsorption of Silk Fibroin Fibers Grafted with Methacrylonitrile" Molecules 28, no. 6: 2551. https://doi.org/10.3390/molecules28062551