Effect of Oxyfluorination of PFA-Coated Metal Mesh with Superhydrophobic Properties on the Filtration Performance of SiO2 Microparticles
Abstract
:1. Introduction
2. Results and Discussion
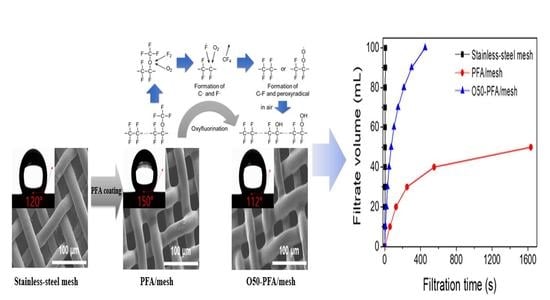
2.1. Morphological Changes on the Surface of the PFA-Coated Mesh
2.2. Surface Analysis
2.3. The Change in the Water Contact Angle
2.4. Water Permeability and Particle Removal Rate
3. Materials and Methods
3.1. Materials
3.2. Stainless-Steel Mesh Coating with PFA Polymer
3.3. Oxyfluorination Reaction
3.4. Surface Energy
3.5. A Test Method for Particle Removal Rate and Water Permeability
3.6. Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, C.Y.; Kao, C.L.; Hung, P.Y. Preparation of activated carbon from waste cation exchange resin and its application in wastewater treatment. Carbon Lett. 2022, 32, 461–474. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Meng, L.Y. Hierarchical porous carbons derived from corncob: Study on adsorption mechanism for gas and wastewater. Carbon Lett. 2021, 31, 643–653. [Google Scholar] [CrossRef]
- Jin, T.; Liu, C.; Chen, F.; Qian, J.; Qiu, Y.; Meng, X.; Chen, Z. Synthesis of g-C3N4/CQDs composite and its photocatalytic degradation property for Rhodamine B. Carbon Lett. 2022, 32, 1451–1462. [Google Scholar] [CrossRef]
- Thue, P.S.; Lima, D.R.; Naushad, M.; Lima, E.C.; Albuquerque, Y.R.T.; Dias, S.L.P.; Cunha, M.R.; Dotto, G.L.; Brum, I.A.S. High removal of emerging contaminants from wastewater by activated carbons derived from the shell of cashew of Para. Carbon Lett. 2021, 31, 13–28. [Google Scholar] [CrossRef]
- Huang, C.J.; Yang, B.M.; Chen, K.S.; Chang, C.C.; Kao, C.M. Application of membrane technology on semiconductor wastewater reclamation: A pilot-scale study. Desalination 2011, 278, 203–210. [Google Scholar] [CrossRef]
- Luna, M.D.; Warmadewanthi, G.; Lui, L.C. Combined treatment of polishing wastewater and fluoride-containing wastewater from a semiconductor manufacturer. Colloids Surf. A 2009, 347, 64–68. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Wu, H.Z.; Ye, M.H.; Chen, J.P.; Huang, H.L.; Lin, P.H.P. An industrial-scale biodegradation system for volatile organics contaminated wastewater from semiconductor manufacturing process. J. Taiwan Inst. Chem. Eng. 2009, 40, 70–76. [Google Scholar] [CrossRef]
- Won, C.H.; Choi, J.; Chung, J. Evaluation of optimal reuse system for hydrofluoric acid wastewater. J. Hazard. Mater. 2012, 239, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kim, E.J.; Koo, B.Y. Effects of wire-type and mesh-type anode current collectors on performance and electrochemistry of microbial fuel cells. Chemosphere 2018, 209, 542–550. [Google Scholar] [CrossRef]
- Huang, J.; Lee, W. Sealing and mechanical behaviors of expanded PTFE gasket sheets characterized by PVRC room temperature tightness tests. Mater. Chem. Phys. 2001, 68, 180–196. [Google Scholar] [CrossRef]
- Godino, M.P.; Pena, L.; Rincón, C.; Mengual, J.I. Water production from brines by membrane distillation. Desalination 1997, 108, 91–97. [Google Scholar] [CrossRef]
- Kar, E.; Bose, N.; Das, S. Submicron graphite platelet-incorporated PVDF composite: An efficient body motion-based energy harvester for flexible electronics. Carbon Lett. 2023. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An Intelligent Superwetting PVDF Membrane Showing Switchable Transport Performance for Oil/Water Separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Díez, L.M.; Florido-Díaz, F.J.; Vázquez-González, M.I. Study of evaporation efficiency in membrane distillation. Desalination 1999, 126, 193–198. [Google Scholar] [CrossRef]
- Brace, K.; Combellas, C.; Dujardin, E.; Thiébault, A.; Delamar, M.; Kanoufi, F.; Shanahan, M.E.R. Surface modification of halogenated polymers: 1. Polytetrafluoroethylene. Polymer 1997, 38, 3295–3305. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chiang, W.Y. Chemical surface treatment of poly(tetrafluoroethylene) powder. Macromol. Mater. Eng. 1993, 209, 9–23. [Google Scholar]
- Long, X.Y.; He, L.F.; Zhang, Y.; Ge, M.Q. Trimethyl borate-treated polytetrafluoroethylene micropowder with improved hydrophilic and adhesive properties based on coordination bond theory. Surf. Interface Anal. 2018, 50, 457–463. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Zhang, S.; Li, J. Preparation and characterization of microencapsulated ammonium polyphosphate with UMF and its application in WPCs. Constr. Build. Mater. 2014, 65, 151–158. [Google Scholar] [CrossRef]
- Badey, J.P.; Espuche, E.; Sage, D.; Chabert, B. A comparative study of the effects of ammonia and hydrogen plasma downstream treatment on the surface modification of polytetrafluoroethylene. Polymer 1996, 37, 1377–1386. [Google Scholar] [CrossRef]
- Lim, C.; Kwak, C.H.; Jeong, S.G.; Kim, D.; Lee, Y.S. Enhanced CO2 adsorption of activated carbon with simultaneous surface etching and functionalization by nitrogen plasma treatment. Carbon Lett. 2023, 33, 139–145. [Google Scholar] [CrossRef]
- Sarani, A.; Geyter, N.D.; Nikiforov, A.Y.; Morent, R.; Leys, C.; Hubert, J.; Reniers, F. Surface modification of PTFE using an atmospheric pressure plasma jet in argon and argon+CO2. Surf. Coat. Technol. 2012, 206, 2226–2232. [Google Scholar] [CrossRef]
- Salapare, H.S.; Guittard, F.; Noblin, X.; Givenchy, E.T.; Celestini, F.; Ramos, H.J. Stability of the hydrophilic and superhydrophobic properties of oxygen plasma- treated poly(tetrafluoroethylene) surfaces. J. Colloid Interface Sci. 2013, 396, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R. Surface modification of poly(tetrafluoroethylene) film by chemical etching, plasma, and ion beam treatments. J. Appl. Polym. Sci. 2000, 77, 1913–1920. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Okazaki, Y.; Shibahara, M.; Nishino, M.; Seto, Y.; Endo, K.; Yamamura, K. Effects of He and Ar Heat-Assisted Plasma Treatments on the Adhesion Properties of Polytetrafluoroethylene (PTFE). Polymers 2021, 13, 4266. [Google Scholar] [CrossRef]
- Primc, G. Recent Advances in Surface Activation of Polytetrafluoroethylene (PTFE) by Gaseous Plasma Treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- Goethem, C.V.; Mertens, M.; Vankelecom, I.F.J. Crosslinked PVDF membranes for aqueous and extreme pH nanofiltration. J. Membr. Sci. 2019, 572, 489–495. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Kang, E.T.; Zhang, Y. Surface Modification of Fluoropolymers via Molecular Design. Adv. Mater. 2000, 12, 1481–1494. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Stolyarov, V.P. Oxyfluorination-Controlled Variations in the Wettability of Polymer Film Surfaces. Colloid J. 2019, 81, 146–157. [Google Scholar] [CrossRef]
- Dane, L.; Rok, Z.; Gregor, P.; Miran, M.; Alenka, V. Optimization of surface wettability of polytetrafluoroethylene (PTFE) by precise dosing of oxygen atoms. Appl. Surf. Sci. 2022, 598, 153817. [Google Scholar]
- Yoo, S.H. Short review of utilization of electron-beam irradiation for preparing polyacrylonitrile-based carbon fibers and improving properties of carbon-fiber-reinforced thermoplastics. Carbon Lett. 2022, 32, 413–429. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nishimura, F.; Kim, J.H.; Yonezawa, S. Dyeable Hydrophilic Surface Modification for PTFE Substrates by Surface Fluorination. Membranes 2023, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, M.J.; Kong, E.Y.; Jeong, J.D.; Lee, Y.S. Effect of Oxyfluorination of Activated Carbon Fibers on Adsorption of Benzene Gas Causing Sick House Syndrome. Appl. Chem. Eng. 2018, 29, 312–317. [Google Scholar]
- Agopian, J.C.; Teraube, O.; Charlet, K.; Dubois, M. A review about the fluorination and oxyfluorination of carbon fibres. J. Fluor. Chem. 2021, 251, 109887. [Google Scholar] [CrossRef]
- Jeong, E.Y.; Bae, T.S.; Yun, S.M.; Woo, S.W.; Lee, Y.S. Surface characteristics of low-density polyethylene films modified by oxyfluorination-assisted graft polymerization. Colloid Surf. A-Physicochem. Eng. Asp. 2011, 373, 36–41. [Google Scholar] [CrossRef]
- Ho, K.K.C.; Lee, A.F.; Bismarck, A. Fluorination of carbon fibres in atmospheric plasma. Carbon 2007, 45, 775–784. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, M.K.; Lee, Y.S. Surface characteristics of fluorine-modified PAN-based carbon fibers. Carbon 2003, 41, 723–730. [Google Scholar] [CrossRef]
- Alentiev, A.Y.; Bogdanova, Y.G.; Vdovichenko, A.Y.; Pashkevich, D.S.; Belov, N.A. Direct Fluorination as Method of Improvement of Operational Properties of Polymeric Materials. Polymers 2020, 12, 2836. [Google Scholar]
- Mahdian, M.; Huang, L.Y.; Kirk, D.W.; Jia, C.Q. Water permeability of monolithic wood biocarbon. Microporous Mesoporous Mat. 2020, 303, 110258. [Google Scholar] [CrossRef]
- Chi, M.; Zheng, P.; Wei, M.; Zhu, A.; Zhong, L.; Zhang, Q.; Liu, Q. Polyamide composite nanofiltration membrane modified by nanoporous TiO2 interlayer for enhanced water permeability. J. Ind. Eng. Chem. 2022, 115, 230–240. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.Y.; Kim, J.W.; Han, J.T.; Lee, Y.S.; Woo, J.S. Influence of oxyfluorinated graphite on fluorinated ethylene–propylene composites as bipolar plates. Carbon Lett. 2020, 30, 345–352. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, S.E.; Lee, Y.S. Improved mechanical and electromagnetic interference shielding properties of epoxy composites through the introduction of oxyfluorinated multiwalled carbon nanotubes. J. Ind. Eng. Chem. 2017, 56, 435–442. [Google Scholar] [CrossRef]
- Jung, M.J.; Ko, Y.Y.; Kim, K.H.; Lee, Y.S. Oxyfluorination of Pitch-based Activated Carbon Fibers for High Power Electric Double Layer Capacitor. Appl. Chem. Eng. 2017, 28, 638–644. [Google Scholar]
- Lim, J.W.; Lee, J.M.; Yun, S.M.; Park, B.J.; Lee, Y.S. Hydrophilic modification of polyacrylonitrile membranes by oxyfluorination. J. Ind. Eng. Chem. 2009, 15, 876–882. [Google Scholar] [CrossRef]
- Yu, H.R.; Kim, J.G.; Im, J.S.; Bae, T.S.; Lee, Y.S. Effects of oxyfluorination on a multi-walled carbon nanotube electrode for a high-performance glucose sensor. J. Ind. Eng. Chem. 2012, 18, 674–679. [Google Scholar] [CrossRef]
- Fujimoto, H.; Maeda, T.; Yoshickwa, M.; Watanabe, N. Physical and chemical properties of pitch fluoride prepared by direct fluorination. J. Fluor. Chem. 1993, 60, 69–77. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, B.K. Surface properties of oxyfluorinated PAN-based carbon fibers. Carbon 2002, 40, 2461–2468. [Google Scholar] [CrossRef]
- Lamperti, R.; Grenfell, J.; Sangiorgi, C.; Lantieri, C.; Airey, G.D. Influence of waxes on adhesion properties of bituminous binders. Constr. Build. Mater. 2015, 76, 404–412. [Google Scholar] [CrossRef]
- Kakar, M.R.; Hamzah, M.O.; Akhtar, M.N.; Woodward, D. Surface free energy and moisture susceptibility evaluation of asphalt binders modified with surfactant-based chemical additive. J. Clean Prod. 2016, 112, 2342–2353. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Song, K.W.; Lee, J.W.; Choi, S.O.; Kim, J.Y. Interaction of Surface Energy Components between Solid and Liquid on Wettability, and Its Application to Textile Anti-Wetting Finish. Polymers 2019, 11, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample | Elemental Content (Atomic %) | ||
|---|---|---|---|
| C | O | F | |
| PFA/mesh | 31.5 | 0.4 | 68.1 |
| O10-PFA/mesh | 31.7 | 0.5 | 67.8 |
| O30-PFA/mesh | 31.4 | 0.5 | 68.1 |
| O50-PFA/mesh | 28.8 | 6.4 | 64.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-S.; Kwak, C.-H.; Ha, S.-M.; Ryu, J.-C.; Lee, Y.-S. Effect of Oxyfluorination of PFA-Coated Metal Mesh with Superhydrophobic Properties on the Filtration Performance of SiO2 Microparticles. Molecules 2023, 28, 3110. https://doi.org/10.3390/molecules28073110
Kim K-S, Kwak C-H, Ha S-M, Ryu J-C, Lee Y-S. Effect of Oxyfluorination of PFA-Coated Metal Mesh with Superhydrophobic Properties on the Filtration Performance of SiO2 Microparticles. Molecules. 2023; 28(7):3110. https://doi.org/10.3390/molecules28073110
Chicago/Turabian StyleKim, Kyung-Soo, Cheol-Hwan Kwak, Seong-Min Ha, Jae-Chun Ryu, and Young-Seak Lee. 2023. "Effect of Oxyfluorination of PFA-Coated Metal Mesh with Superhydrophobic Properties on the Filtration Performance of SiO2 Microparticles" Molecules 28, no. 7: 3110. https://doi.org/10.3390/molecules28073110