CoO–Co Heterojunction Covered with Carbon Enables Highly Efficient Integration of Hydrogen Evolution and 5-Hydroxymethylfurfural Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterizations of CoO–Co@C/CF
2.2. Electrochemical Activity of CoO–Co@C/CF towards HMF Oxidation
2.3. Electrochemical Activity of CoO–Co@C/CF towards HER
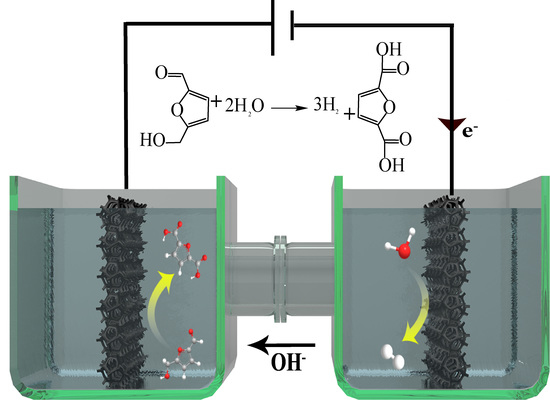
2.4. Integrated HMF Oxidation and H2 Evolution on CoO–Co@C/CF
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of the Electrodes
3.3. Characterizations
3.4. Electrochemical Measurements
3.5. Product Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Luo, Q.M.; Zhao, Y.W.; Sun, L.; Wang, C.; Xin, H.Q.; Song, J.X.; Li, D.Y.; Ma, F. Interface oxygen vacancy enhanced alkaline hydrogen evolution activity of cobalt-iron phosphide/CeO2 hollow nanorods. Chem. Eng. J. 2022, 437, 135376. [Google Scholar] [CrossRef]
- Li, Q.; Huang, F.; Li, S.; Zhang, H.; Yu, X.Y. Oxygen Vacancy Engineering Synergistic with Surface Hydrophilicity Modification of Hollow Ru Doped CoNi-LDH Nanotube Arrays for Boosting Hydrogen Evolution. Small 2022, 18, e2104323. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.Y.; Zhang, G.Q. A general strategy for constructing transition metal Oxide/CeO2 heterostructure with oxygen vacancies toward hydrogen evolution reaction and oxygen evolution reaction. J. Power Sources 2021, 512, 230514. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, H.; Liu, Y.; Mo, J.; Wang, M.; Li, J.; Tian, X. Exploring and understanding the internal voltage losses through catalyst layers in proton exchange membrane water electrolysis devices. Appl. Energy 2022, 317, 119213. [Google Scholar] [CrossRef]
- Manivelan, N.; Karuppanan, S.; Prabakar, K. Djurleite Copper Sulfide-Coupled Cobalt Sulfide Interface for a Stable and Efficient Electrocatalyst. ACS Appl. Mater. Interfaces 2022, 14, 30812–30823. [Google Scholar] [CrossRef] [PubMed]
- Prabu, N.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. Bio-derived nanoporous activated carbon sheets as electrocatalyst for enhanced electrochemical water splitting. Int. J. Hydrog. Energy 2019, 44, 19995–20006. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Li, R.; Rao, P.; Li, J.; Deng, P.; Luo, J.; Huang, W.; Chen, Q.; Kang, Z.; et al. Engineering the strong metal support interaction of titanium nitride and ruthenium nanorods for effective hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 317, 121796. [Google Scholar] [CrossRef]
- Feng, S.; Luo, J.; Li, J.; Yu, Y.; Kang, Z.; Huang, W.; Chen, Q.; Deng, P.; Shen, Y.; Tian, X. Heterogeneous structured Ni3Se2/MoO2@Ni12P5 catalyst for durable urea oxidation reaction. Mater. Today Phys. 2022, 23, 100646. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Song, Y.; Zhou, W.; Shao, Z. Designing High-Valence Metal Sites for Electrochemical Water Splitting. Adv. Func. Mater. 2021, 31, 2009779. [Google Scholar] [CrossRef]
- Mai, H.D.; Le, V.C.T.; Yoo, H. Effective Fabrication and Electrochemical Oxygen Evolution Reaction Activity of Gold Multipod Nanoparticle Core-Cobalt Sulfide Shell Nanohybrids. ACS Appl. Nano Mater. 2019, 2, 678–688. [Google Scholar] [CrossRef]
- Swain, S.; Altaee, A.; Saxena, M.; Samal, A.K. A comprehensive study on heterogeneous single atom catalysis: Current progress, and challenges. Coordin. Chem. Rev. 2022, 470, 214710. [Google Scholar] [CrossRef]
- Rajapriya, A.; Keerthana, S.; Rebekah, A.; Viswanathan, C.; Ponpandian, N. Enriched oxygen vacancy promoted heteroatoms (B, P, N, and S) doped CeO2: Challenging electrocatalysts for oxygen evolution reaction (OER) in alkaline medium. Int. J. Hydrog. Energy 2021, 46, 37281–37293. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Qi, Y.F.; Li, W.J.; Wang, C. Efficient Electrooxidation of 5-Hydroxymethylfurfural Using Co-Doped Ni3S2 Catalyst: Promising for H2 Production under Industrial-Level Current Density. Adv. Sci. 2022, 9, 2200957. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Xie, W.F.; Song, Y.J.; Li, H.; Li, S.J.; Jiang, S.; Lee, J.Y.; Shao, M.F. Bifunctional integrated electrode for high-efficient hydrogen production coupled with 5-hydroxymethylfurfural oxidation. Appl. Catal. B Environ. 2022, 312, 121400. [Google Scholar] [CrossRef]
- Zheng, R.J.; Zhao, C.H.; Xiong, J.H.; Teng, X.; Chen, W.H.; Hu, Z.B.; Chen, Z.F. Construction of a hierarchically structured, NiCo-Cu-based trifunctional electrocatalyst for efficient overall water splitting and 5-hydroxymethylfurfural oxidation. Sustain. Energy Fuels 2021, 5, 4023–4031. [Google Scholar] [CrossRef]
- Park, M.; Gu, M.S.; Kim, B.S. Tailorable Electrocatalytic 5-Hydroxymethylfurfural Oxidation and H2 Production: Architecture-Performance Relationship in Bifunctional Multilayer Electrodes. ACS Nano 2020, 14, 6812–6822. [Google Scholar] [CrossRef]
- You, B.; Jiang, N.; Liu, X.; Sun, Y. Simultaneous H2 Generation and Biomass Upgrading in Water by an Efficient Noble-Metal-Free Bifunctional Electrocatalyst. Angew Chem. Int. Ed. Engl. 2016, 55, 9913–9917. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, C.L.; Huang, Y.C.; Zou, Y.; Liu, Z.; Liu, Y.; Li, Y.; He, N.; Shi, J.; Wang, S. Identifying the Geometric Site Dependence of Spinel Oxides for the Electrooxidation of 5-Hydroxymethylfurfural. Angew Chem. Int. Ed. Engl. 2020, 59, 19215–19221. [Google Scholar] [CrossRef]
- Luo, R.P.; Li, Y.Y.; Xing, L.X.; Zhong, R.Y.; Qian, Z.Y.; Yin, G.P.; Wang, Y.C.; Du, L. A dynamic Ni(OH)2-NiOOH/NiFeP heterojunction enabling high-performance E-upgrading of hydroxymethylfurfural. Appl. Catal. B Environ. 2022, 311, 121357. [Google Scholar] [CrossRef]
- Zeng, J.R.; Chen, W.H.; Zhang, G.W.; Yu, L.; Zhong, L.B.A.; Liu, Y.; Zhao, S.F.; Qiu, Y.J. Heterostructured Ni3N-NiMoN Nanowires as Bifunctional Electrocatalysts for Hydrogen Evolution and 5-Hydroxymethylfurfural Oxidation. ACS Appl. Nano Mater. 2022, 5, 7321–7330. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.Q.; Liu, B.Y.; Chen, Z.; Xu, H.; Yan, K. Trimetallic NiCoFe-Layered Double Hydroxides Nanosheets Efficient for Oxygen Evolution and Highly Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural. ACS Catal. 2020, 10, 5179–5189. [Google Scholar] [CrossRef]
- Yin, Z.; Pang, H.; Guo, X.; Lin, H.; Muzzio, M.; Shen, M.; Wei, K.; Yu, C.; Williard, P.; Sun, S. CuPd Nanoparticles as a Robust Catalyst for Electrochemical Allylic Alkylation. Angew Chem. Int. Ed. Engl. 2020, 59, 15933–15936. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Sun, X.; Yao, Z.H.; Zhong, X.; Coo, Y.Y.; Liang, Y.L.; Wei, Z.Z.; Deng, S.W.; Zhuang, G.L.; Li, X.N.; et al. Biomass Valorization via Paired Electrosynthesis Over Vanadium Nitride-Based Electrocatalysts. Adv. Func. Mater. 2019, 29, 1904780. [Google Scholar] [CrossRef]
- Deng, X.H.; Kang, X.M.; Li, M.; Xiang, K.; Wang, C.; Guo, Z.P.; Zhang, J.J.; Fu, X.Z.; Luo, J.L. Coupling efficient biomass upgrading with H2 production via bifunctional CuxS@NiCo-LDH core-shell nanoarray electrocatalysts. J. Mater. Chem. A 2020, 8, 1138–1146. [Google Scholar] [CrossRef]
- Zhao, G.C.; Hai, G.T.; Zhou, P.Y.; Liu, Z.M.; Zhang, Y.Y.; Peng, B.X.; Xia, W.; Huang, X.B.; Wang, G. Electrochemical Oxidation of 5-Hydroxymethylfurfural on CeO2-Modified Co3O4 with Regulated Intermediate Adsorption and Promoted Charge Transfer. Adv. Funct. Mater. 2023, 33, 2213170. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhou, Y.M.; Tao, S.Y. CoP-CoOOH heterojunction with modulating interfacial electronic structure: A robust biomass-upgrading electrocatalyst. Appl. Catal. B Environ. 2022, 315, 121588. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, C.; An, J.T.; Zhuang, Y.; Tao, S.Y. Surface reconstruction of NiCoP for enhanced biomass upgrading. J. Mater. Chem. A 2021, 9, 18421–18430. [Google Scholar] [CrossRef]
- Liu, H.Z.; Lee, T.H.; Chen, Y.F.; Cochran, E.W.; Li, W.Z. Paired and Tandem Electrochemical Conversion of 5-(Hydroxymethyl)furfural Using Membrane-Electrode Assembly-Based Electrolytic Systems. Chemelectrochem 2021, 8, 2817–2824. [Google Scholar] [CrossRef]
- Fan, L.F.; Ji, Y.X.; Wang, G.X.; Zhang, Z.F.; Yi, L.C.; Chen, K.; Liu, X.; Wen, Z.H. Bifunctional Mn-doped CoSe2 nanonetworks electrode for hybrid alkali/acid electrolytic H2 generation and glycerol upgrading. J. Energy Chem. 2022, 72, 424–431. [Google Scholar] [CrossRef]
- Yang, S.W.; Guo, Y.; Zhao, Y.K.; Zhang, L.; Shen, H.D.; Wang, J.H.; Li, J.J.; Wu, C.; Wang, W.B.; Cao, Y.L.; et al. Construction of Synergistic Ni3S2-MoS2 Nanoheterojunctions on Ni Foam as Bifunctional Electrocatalyst for Hydrogen Evolution Integrated with Biomass Valorization. Small 2022, 18, 2201306. [Google Scholar] [CrossRef]
- Yang, L.J.; Yu, Y.; Zhang, L. Construction of Dual-functional 2D/3D Hydrid Co2P-CeOx Heterostructure Integrated Electrode for Electrocatalytic Urea Oxidation Assisted Hydrogen Production. Chem. J. Chin. U 2022, 43, 20220082. [Google Scholar] [CrossRef]
- Lei, L.; Yin, Z.; Huang, D.L.; Chen, Y.S.; Chen, S.; Cheng, M.; Du, L.; Liang, Q.H. Metallic Co and crystalline Co-Mo oxides supported on graphite felt for bifunctional electrocatalytic hydrogen evolution and urea oxidation. J. Colloid Interf. Sci. 2022, 612, 413–423. [Google Scholar] [CrossRef]
- Sriram, S.; Mathi, S.; Vishnu, B.; Jayabharathi, J. Entwined Co(OH)2 In Situ Anchoring on 3D Nickel Foam with Phenomenal Bifunctional Activity in Overall Water Splitting. Energy Fuel 2022, 36, 7006–7016. [Google Scholar] [CrossRef]
- Yang, C.M.; Wang, C.T.; Zhou, L.H.; Duan, W.; Song, Y.Y.; Zhang, F.C.; Zhen, Y.Z.; Zhang, J.J.; Bao, W.W.; Lu, Y.X.; et al. Refining d-band center in Ni0.85Se by Mo doping: A strategy for boosting hydrogen generation via coupling electrocatalytic oxidation 5-hydroxymethylfurfural. Chem. Eng. J. 2021, 422, 130125. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- He, X.B.; Yin, F.X.; Yuan, S.; Liu, N.; Huang, X.F. Hybrid Spinel Oxides/N-Doped Reduced Graphene Oxide as Highly-Active Bifunctional Electrocatalysts for Oxygen Reduction/Evolution Reactions. Chemelectrochem 2016, 3, 1107–1115. [Google Scholar] [CrossRef]
- Hanan, A.; Shu, D.; Aftab, U.; Cao, D.X.; Laghari, A.J.; Solangi, M.Y.; Abro, M.I.; Nafady, A.; Vigolo, B.; Tahira, A.; et al. Co2FeO4@rGO composite: Towards trifunctional water splitting in alkaline media. Int. J. Hydrog. Energy 2022, 47, 33919–33937. [Google Scholar] [CrossRef]
- Yang, H.Q.; Wang, B.D.; Kou, S.Q.; Lu, G.L.; Liu, Z.N. Mott-Schottky heterojunction of Co/Co2P with built-in electric fields for bifunctional oxygen electrocatalysis and zinc-air battery. Chem. Eng. J. 2021, 425, 131589. [Google Scholar] [CrossRef]
- Li, S.S.; Zhang, Y.; Yuan, Y.; Chang, F.F.; Zhu, K.; Li, G.; Bai, Z.Y.; Yang, L. Design and synthesis of dispersed Ni2P/Co nano heterojunction as bifunctional electrocatalysis for boosting overall water splitting. Int. J. Hydrog. Energy 2023, 48, 3355–3363. [Google Scholar] [CrossRef]
- Sun, H.C.; Yang, J.M.; Li, J.G.; Li, Z.S.; Ao, X.; Liu, Y.Z.; Zhang, Y.; Li, Y.; Wang, C.D.; Tang, J. Synergistic coupling of NiTe nanoarrays with RuO2 and NiFe-LDH layers for high-efficiency electrochemical-/photovoltage-driven overall water splitting. Appl. Catal. B Environ. 2020, 272, 118988. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Jiang, N.; Sun, Y.J. A General Strategy for Decoupled Hydrogen Production from Water Splitting by Integrating Oxidative Biomass Valorization. J. Am. Chem. Soc. 2016, 138, 13639–13646. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zhai, X.W.; Fan, C.C.; Chen, X.X.; Liu, Y.L.; Yang, J.M.; Chen, L.; Ge, G.X.; Zhang, J.L. Hybrid-metal hydroxyl fluoride nanosheet arrays as a bifunctional electrocatalyst for efficient overall water splitting. J. Mater. Chem. A 2022, 10, 11774–11783. [Google Scholar] [CrossRef]
- Ren, Y.F.; Wang, C.T.; Duan, W.; Zhou, L.H.; Pang, X.X.; Wang, D.J.; Zhen, Y.Z.; Yang, C.M.; Gao, Z.W. MoS2/Ni3S2 Schottky heterojunction regulating local charge distribution for efficient urea oxidation and hydrogen evolution. J. Colloid Interf. Sci. 2022, 628, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Cao, X.; Ding, G.F.; Guo, Z.X.; Xue, Y.Y.; Li, G.H.; Yu, R.H. Constructing oxygen vacancy-enriched Fe2O3@NiO heterojunctions for highly efficient electrocatalytic alkaline water splitting. Crystengcomm 2022, 24, 199–207. [Google Scholar] [CrossRef]
- Hu, J.; Al-Salihy, A.; Wang, J.; Li, X.; Fu, Y.F.; Li, Z.H.; Han, X.J.; Song, B.; Xu, P. Improved Interface Charge Transfer and Redistribution in CuO-CoOOH p-n Heterojunction Nanoarray Electrocatalyst for Enhanced Oxygen Evolution Reaction. Adv. Sci. 2021, 8, 2103314. [Google Scholar] [CrossRef]
- Zhou, M.J.; Chen, J.M.; Li, Y.W. CoP nanorods anchored on Ni2P-NiCoP nanosheets with abundant heterogeneous interfaces boosting the electrocatalytic oxidation of 5-hydroxymethyl-furfural. Catal. Sci. Tech. 2022, 12, 4288–4297. [Google Scholar] [CrossRef]
- Xiang, S.; Dong, L.; Wang, Z.Q.; Han, X.; Daemen, L.L.; Li, J.; Cheng, Y.; Guo, Y.; Liu, X.; Hu, Y.; et al. A unique Co@CoO catalyst for hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran. Nat. Commun. 2022, 13, 3657. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, T.; Dong, C.L.; Huang, Y.C.; Li, Y.; Chen, J.; Zou, Y.; Wang, S. Tuning the Selective Adsorption Site of Biomass on Co3O4 by Ir Single Atoms for Electrosynthesis. Adv. Mater. 2021, 33, e2007056. [Google Scholar] [CrossRef]
- Gao, L.; Gan, S.; Ma, J.; Sun, Z.; Liu, Z.; Zhong, L.; Zhou, K.; Han, F.; Wang, W.; Han, D.; et al. Titanium Oxide-Confined Manganese Oxide for One-Step Electrocatalytic Preparation of 2,5-Furandicarboxylic Acid in Acidic Media. Chemelectrochem 2020, 7, 4251–4258. [Google Scholar] [CrossRef]
- Kang, M.J.; Park, H.; Jegal, J.; Hwang, S.Y.; Kang, Y.S.; Cha, H.G. Electrocatalysis of 5-hydroxymethylfurfural at cobalt based spinel catalysts with filamentous nanoarchitecture in alkaline media. Appl. Catal. B: Environ. 2019, 242, 85–91. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, C.; Gao, M.; Xia, B.; Zhang, J. In situ anchoring of a Co3O4 nanowire on nickel foam: An outstanding bifunctional catalyst for energy-saving simultaneous reactions. Green Chem. 2019, 21, 6699–6706. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.T.; Yao, Y.; Zhang, Z.H.; Yang, Z.Z.; Li, J.; Chen, K.Q.; Lu, X.Y.; Ouyang, P.K.; Fu, J. Cu-Ni Bimetallic Hydroxide Catalyst for Efficient Electrochemical Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Chemelectrochem 2019, 6, 5797–5801. [Google Scholar] [CrossRef]
- Taitt, B.J.; Nam, D.-H.; Choi, K.-S. A Comparative Study of Nickel, Cobalt, and Iron Oxyhydroxide Anodes for the Electrochemical Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Catal. 2019, 9, 660–670. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Z.; Ma, J.; Zhong, L.; Song, Z.; Xu, J.; Gan, S.; Han, D.; Niu, L. NiSe@NiOx core-shell nanowires as a non-precious electrocatalyst for upgrading 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid. Appl. Catal. B: Environ. 2020, 261, 118235. [Google Scholar] [CrossRef]
- Zhang, N.; Zou, Y.; Tao, L.; Chen, W.; Zhou, L.; Liu, Z.; Zhou, B.; Huang, G.; Lin, H.; Wang, S. Electrochemical Oxidation of 5-Hydroxymethylfurfural on Nickel Nitride/Carbon Nanosheets: Reaction Pathway Determined by In Situ Sum Frequency Generation Vibrational Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 15895–15903. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, H.; Mu, T. Hierarchical NiSx/Ni2P nanotube arrays with abundant interfaces for efficient electrocatalytic oxidation of 5-hydroxymethylfurfural. Green Chem. 2022, 24, 877–884. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Z.; Zhao, X.; Zhang, B.; Mu, T. Controllable and facile preparation of Co9S8–Ni3S2 heterostructures embedded with N,S,O-tri-doped carbon for electrocatalytic oxidation of 5-hydroxymethylfurfural. Green Chem. 2022, 24, 1721–1731. [Google Scholar] [CrossRef]
- Wang, C.; Bongard, H.-J.; Yu, M.; Schüth, F. Highly Ordered Mesoporous Co3O4 Electrocatalyst for Efficient, Selective, and Stable Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Chemsuschem 2021, 14, 5199–5206. [Google Scholar] [CrossRef]
- Zhong, Y.; Ren, R.-Q.; Qin, L.; Wang, J.-B.; Peng, Y.-Y.; Li, Q.; Fan, Y.-M. Electrodeposition of hybrid nanosheet-structured NiCo2O4 on carbon fiber paper as a non-noble electrocatalyst for efficient electrooxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. New J. Chem. 2021, 45, 11213–11221. [Google Scholar] [CrossRef]
- Tao, Y.; Fan, S.; Li, X.; Yang, J.; Wang, J.; Tadé, M.O.; Liu, S. CuxCo3–xO4 Spinel Nanofibers for Selective Oxidation of 5-Hydroxymethylfurfural into Fuel Additives. ACS Appl. Nano Mater. 2022, 5, 16564–16572. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y. In situ Cobalt–Cobalt Oxide/N-Doped Carbon Hybrids As Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Zang, J.B.; Chen, L.; Chen, L.B.; Chen, X.; Wu, P.; Zhou, S.Y.; Wang, Y.H. A microwave-assisted synthesis of CoO@Co core–shell structures coupled with N-doped reduced graphene oxide used as a superior multi-functional electrocatalyst for hydrogen evolution, oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2017, 5, 5865–5872. [Google Scholar] [CrossRef]
- Pei, Z.; Tang, Z.; Liu, Z.; Huang, Y.; Wang, Y.; Li, H.; Xue, Q.; Zhu, M.; Tang, D.; Zhi, C. Construction of a hierarchical 3D Co/N-carbon electrocatalyst for efficient oxygen reduction and overall water splitting. J. Mater. Chem. A 2018, 6, 489–497. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Hao, X.-F.; Jiang, Z.; Sun, X.-P.; Xu, D.; Wang, J.; Zhong, H.-X.; Meng, F.-L.; Zhang, X.-B. C and N Hybrid Coordination Derived Co–C–N Complex as a Highly Efficient Electrocatalyst for Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 15070–15073. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ha, Y.; Jia, H.; Yan, X.; Chen, M.; Liu, M.; Wu, R. Water Splitting: Oriented Transformation of Co-LDH into 2D/3D ZIF-67 to Achieve Co–N–C Hybrids for Efficient Overall Water Splitting. Adv. Energ. Mater. 2019, 9, 1970066. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Ren, P.; Deng, D.; Bao, X. Enhanced Electron Penetration through an Ultrathin Graphene Layer for Highly Efficient Catalysis of the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2100–2104. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Liu, C.; Ye, M.; Yang, Q.; Liu, Z.; Xie, L.; Yang, G. Enhanced electron transport through a nanoforest-like structure of CoNi nanoalloy@nitrogen-doped carbon nanotubes for highly efficient catalysis of overall water splitting. Appl. Surf. Sci. 2020, 517, 145841. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, P.; Hua, X.; Luo, W.; Cheng, G.; Xing, W.; Chen, S. A cobalt-based hybrid electrocatalyst derived from a carbon nanotube inserted metal–organic framework for efficient water-splitting. J. Mater. Chem. A 2016, 4, 16057–16063. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, N.; Zhang, L.; Yin, L.; Zhao, Y.; Zhang, B. Self-supported Co/CoO anchored on N-doped carbon composite as bifunctional electrocatalyst for efficient overall water splitting. Chem. Eng. J. 2021, 414, 128804. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, H.; Smith, R.L.; Qi, X. Electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid via metal-organic framework-structured hierarchical Co3O4 nanoplate arrays. J. Colloid Interf. Sci. 2023, 632, 87–94. [Google Scholar] [CrossRef]
- Aftab, U.; Tahira, A.; Mazzaro, R.; Morandi, V.; Ishaq Abro, M.; Baloch, M.M.; Yu, C.; Ibupoto, Z.H. Nickel–cobalt bimetallic sulfide NiCo2S4 nanostructures for a robust hydrogen evolution reaction in acidic media. RSC Adv. 2020, 10, 22196–22203. [Google Scholar] [CrossRef]
- Bai, X.-J.; Chen, H.; Li, Y.-N.; Shao, L.; Ma, J.-C.; Li, L.-L.; Chen, J.-Y.; Wang, T.-Q.; Zhang, X.-M.; Zhang, L.-Y.; et al. CoNi-based metal–organic framework nanoarrays supported on carbon cloth as bifunctional electrocatalysts for efficient water-splitting. New J. Chem. 2020, 44, 1694–1698. [Google Scholar] [CrossRef]
- Song, N.; Hong, S.; Xiao, M.; Zuo, Y.; Jiang, E.; Li, C.; Dong, H. Fabrication of Co(Ni)-P surface bonding states on core–shell Co(OH)2@P-NiCo-LDH towards electrocatalytic hydrogen evolution reaction. J. Colloid Interf. Sci. 2021, 582, 535–542. [Google Scholar] [CrossRef]
- Jin, W.; Chen, J.; Wu, H.; Zang, N.; Li, Q.; Cai, W.; Wu, Z. Interface engineering of oxygen-vacancy-rich NiCo2O4/NiCoP heterostructure as an efficient bifunctional electrocatalyst for overall water splitting. Catal. Sci. Tech. 2020, 10, 5559–5565. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, X.; Zhong, X.; Li, S.; Liu, T.; Zhuang, G.; Li, X.; Deng, S.; Mei, D.; Wang, J.-G. Hierarchical Porous NC@CuCo Nitride Nanosheet Networks: Highly Efficient Bifunctional Electrocatalyst for Overall Water Splitting and Selective Electrooxidation of Benzyl Alcohol. Adv. Func. Mater. 2017, 27, 1704169. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Lang, C.-C.; Gao, M.-R.; Chen, Y.; Fu, Q.-Q.; Duan, Y.; Yu, S.-H. Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energ. Environ. Sci. 2018, 11, 1890–1897. [Google Scholar] [CrossRef]
- Huang, Y.; Chong, X.; Liu, C.; Liang, Y.; Zhang, B. Boosting Hydrogen Production by Anodic Oxidation of Primary Amines over a NiSe Nanorod Electrode. Angew. Chem. Int. Ed. 2018, 57, 13163–13166. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Z.; Li, Q.; Jin, W.; Wu, Z.; Fu, G.; Liu, X. Ni-foam supported Co(OH)F and Co–P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J. Mater. Chem. A 2019, 7, 3697–3703. [Google Scholar] [CrossRef]
- Li, M.; Deng, X.; Xiang, K.; Liang, Y.; Zhao, B.; Hao, J.; Luo, J.-L.; Fu, X.-Z. Value-Added Formate Production from Selective Methanol Oxidation as Anodic Reaction to Enhance Electrochemical Hydrogen Cogeneration. Chemsuschem 2020, 13, 914–921. [Google Scholar] [CrossRef]
- Yang, G.; Jiao, Y.; Yan, H.; Xie, Y.; Wu, A.; Dong, X.; Guo, D.; Tian, C.; Fu, H. Interfacial Engineering of MoO2-FeP Heterojunction for Highly Efficient Hydrogen Evolution Coupled with Biomass Electrooxidation. Adv Mater 2020, 32, e2000455. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Du, S.; Gong, R.; Jia, W.; Chen, Z.; Ren, Z. CoO–Co Heterojunction Covered with Carbon Enables Highly Efficient Integration of Hydrogen Evolution and 5-Hydroxymethylfurfural Oxidation. Molecules 2023, 28, 3040. https://doi.org/10.3390/molecules28073040
Zhao L, Du S, Gong R, Jia W, Chen Z, Ren Z. CoO–Co Heterojunction Covered with Carbon Enables Highly Efficient Integration of Hydrogen Evolution and 5-Hydroxymethylfurfural Oxidation. Molecules. 2023; 28(7):3040. https://doi.org/10.3390/molecules28073040
Chicago/Turabian StyleZhao, Lei, Shichao Du, Rui Gong, Wanqi Jia, Zhimin Chen, and Zhiyu Ren. 2023. "CoO–Co Heterojunction Covered with Carbon Enables Highly Efficient Integration of Hydrogen Evolution and 5-Hydroxymethylfurfural Oxidation" Molecules 28, no. 7: 3040. https://doi.org/10.3390/molecules28073040