Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities
Abstract
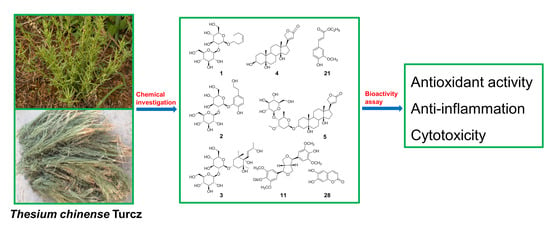
:1. Introduction
2. Results and Discussion
2.1. Structure Identification
2.2. Bio-Activities of Compounds 2–29
2.2.1. Antioxidant Activity
2.2.2. Anti-Inflammatory Activity
2.2.3. Cytotoxic Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
Characterization of the Isolated Compounds 1, 2, 3:
3.4. DPPH Radical Scavenging Assay
3.5. Anti-Inflammatory Activity
3.6. Cytotoxic Assay (CCK-8 Assay)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickrent, D.L.; García, M.A. Lacomucinaea, a new monotypic genus in Thesiaceae (Santalales). Phytotaxa 2015, 224, 173–184. [Google Scholar] [CrossRef]
- Editorial Board of Flora of China. Flora of China; Science Press: Beijing, China, 1988; pp. 76–80. [Google Scholar]
- Li, G.H.; Fang, K.L.; Yang, K.; Cheng, X.P.; Wang, X.N.; Shen, T.; Lou, H.X. Thesium chinense Turcz.: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 273, 113950. [Google Scholar] [CrossRef]
- Lombard, N.; Stander, M.A.; Redelinghuys, H.; Le-Roux, M.M.; Van-Wyk, B.E. A Study of Phenolic Compounds and Their Chemophenetic Value in the Genus Thesium (Santalaceae). Diversity 2022, 14, 590. [Google Scholar] [CrossRef]
- Lombard, N.; Van-Wyk, B.E.; Le-Roux, M.M. A review of the ethnobotany, contemporary uses, chemistry and pharmacology of the genus Thesium (Santalaceae). J. Ethnopharmacol. 2020, 256, 112745. [Google Scholar] [CrossRef]
- Parveen, Z.; Deng, Y.; Saeed, M.K.; Dai, R.; Ahamad, W.; Yu, Y.H. Antiinflammatory and analgesic activities of Thesium chinense Turcz extracts and its major flavonoids, kaempferol and kaempferol-3-O-glucoside. Yakugaku Zasshi 2007, 127, 1275–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Long, Z.; Xu, X.; Wang, L.; Ying, M. Comparison of wild and cultured Thesium chinense Turcz on bacteriostasis and anti-inflammation. Chin. J. Pharm. Biotec. 2006, 13, 219–222. [Google Scholar]
- Ding, X.; Zhang, S.; Ming, L. Analgesic effect of Bairui buccal tablet on mice. J. Huaihai Med. 2001, 1, 17–18. [Google Scholar]
- Shao, L.; Sun, Y.; Liang, J.; Li, M.; Li, X. Decolorization affects the structural characteristics and antioxidant activity of polysaccharides from Thesium chinense Turcz: Comparison of activated carbon and hydrogen peroxide decolorization. Int. J. Biol. Macromol. 2020, 155, 1084–1091. [Google Scholar] [CrossRef]
- Xuan, W.; Tang, D.; Bian, J.; Hu, S.; Hu, J.; Fan, Z. Experimental study on therapeutic effect of Thesium chinense on adriamycin induced nephropathy in rats. J. Pharm. Pract. 2012, 30, 443–446. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Version C.01. Software for NMR Computation, Gaussian Inc.: Wallingford, CT, USA, 2022.
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Sugiyama, M.; Kikuchi, M. Studies on the Constituents of Osmanthus Species. X. Structures of phenolic glucosides from the leaves of Osmanthus asiaticus Nakai. Chem. Pharm. Bull. 1992, 40, 325–326. [Google Scholar] [CrossRef] [Green Version]
- Kiichiro, K.; Masao, H.; Tsutomu, F. Biotransformation of digitoxigenin by cell suspension cultures of Strophanthus amboensis. Phytochemistry 1988, 27, 3475–3479. [Google Scholar]
- Seo, S.; Tomita, Y.; Tori, K. Biosynthesis of oleanene- and ursene-type triterpenes from [4-13C] mevalonic acid in tissue cultures of Isodon japonicus Hara. J. Chem. Soc. Chem. Commun. 1875, 6, 270–271. [Google Scholar] [CrossRef]
- Saracoglu, I.; Varel, M.; Harput, U.S.; Nagatsu, A. Acylated flavonoids and phenol glycosides from Veronica thymoides subsp. Pseudocinerea. Phytochemistry 2004, 65, 2379–2385. [Google Scholar] [CrossRef]
- Yin, J.G.; Yuan, C.S.; Jia, Z.J. A new iridoid and other chemical constituents from Pedicularis kansuensis forma albiflora Li. Arch. Pharm. Res. 2007, 30, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.H.; Dai, Y.; Wong, M.S.; Yao, X.S. New lignans from the bioactive fraction of Sambucus williamsii Hance and proliferation activities on osteoblastic-like UMR106 cells. Fitoterapia 2014, 94, 29–35. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Hisada, S.; Nishide, S. Lignans from bark of Fraxinus mandshurica var. japonica and F. japonica. Chem. Pharm. Bull. 1984, 32, 4482–4489. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wu, W.; Gan, G.; Wang, D.; Gong, J.; Fang, K.; Lu, F. (-)-Syringaresinol-4-O-beta-D-glucopyranoside from Cortex Albizziae inhibits corticosterone-induced PC12 cell apoptosis and relieves the associated dysfunction. Food Chem. Toxicol. 2020, 141, 111394. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, H.; Yaoita, Y.; Kikuchi, M. Isolation and absolute structures of the neolignan glycosides with the enantiometric aglycones from the leaves of Viburnum awabuki K Koch. Chem. Pharm. Bull. 1996, 44, 1122–1123. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, M.; Warashina, T.; Noro, T. Bufadienolides and a new lignan from the bulbs of Urginea maritima. Chem. Pharm. Bull. 2001, 49, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Yin, C.; Cheng, Z. A new tetrahydrofuran lignan diglycoside from Viola tianshanica Maxim. Molecules 2013, 18, 13636–13644. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; He, F.; Ma, Q.L.; Chen, J.; Aisa, H.A. Chemical constituents of Artemisia rupestris. Chem. Nat. Comp. 2017, 53, 991–993. [Google Scholar] [CrossRef]
- Li, L.; Seeram, N.P. Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals. J. Agric. Food. Chem. 2011, 59, 7708–7716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greca, M.D.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Sugiyama, M.; Kikuchi, M. Characterization of lariciresinol glucosides from Osmanthus asiaticus. Heterocycles 1993, 36, 117–121. [Google Scholar]
- Li, Y.C.; Kuo, Y.H. Four new compounds, ficusal, ficusesquilignan A, B, and ficusolide diacetate from the heartwood of Ficus microcarpa. Chem. Pharm. Bull. 2000, 48, 1862–1865. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Escobedo, L.; Gotor-Fernández, V. Solvent role in the lipase-catalysed esterification of cinnamic acid and derivatives, optimisation of the biotransformation conditions. Tetrahedron 2021, 81, 131873. [Google Scholar] [CrossRef]
- Alavi, S.H.R.; Yassa, N.; Hajiaghaee, R.; Yekta, M.M.; Ashtiani, N.R.; Ajani, Y.; Hadjiakhondi, A. Phenolic compounds from Peucedanum ruthenicum M. Bieb, Iran. J. Pharm. Res. 2009, 8, 71–75. [Google Scholar]
- Kamto, E.L.D.; Ngono, D.S.B.; Mbing, J.N.; Atchadé, A.T.; Pegnyemb, D.E.; Westhuizen, J.H. An aromatic amide C-glycoside and a cyclitol derivative from stem barks of Piper guineense Schum and Thonn (Piperaceae). Phytochem. Lett. 2014, 10, 76–81. [Google Scholar] [CrossRef]
- Liu, L.; Zou, M.; Yin, Q.; Zhang, Z.; Zhang, X. Phenylpropanoids from Liparis nervosa and their in vitro antioxidant and α-glucosidase inhibitory activities. Med. Chem. Res. 2021, 30, 1005–1010. [Google Scholar] [CrossRef]
- Sticher, O.; Lahloub, M.F. Phenolic glycosides of Paulownia tomentosa bark. Planta Med. 1982, 46, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ha, S.K.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Phenolic Constituents from the twigs of Euonymus alatus and their cytotoxic and anti-inflammatory activity. Planta Med. 2013, 79, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Zang, E.H.; Chen, Z.W.; Zhang, C.H.; Li, M.H. Chemical constituents of Physochlaina physaloides (L.) G. Don (Solanaceae). Biochem. Syst. Ecol. 2021, 98, 104332. [Google Scholar] [CrossRef]
- Wang, C.; Chao, Z.; Sun, W.; Wu, X.; Ito, Y. Isolation of glycosides from the barks of Ilex Rotunda by high-speed counter-current chromatography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2363–2376. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, Y.Y.; Gao, Z.Q.; Chen, D.; Liu, G.; Wan, B.B.; Jiang, F.J.; Wei, M.X.; Zuo, J.; Zhu, J.; et al. Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 2069–2081. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, L.; Zhang, Z.; Wei, C.X.; Gong, G.H. Ethyl ferulate contributes to the inhibition of the inflammatory responses in murine RAW 264.7 macrophage cells and acute lung injury in mice. PLoS ONE 2021, 16, e0251578. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, H.; Priyanka. Formation of nitrogen-containing six-membered heterocycles on steroidal ring system: A review. Steroids 2022, 191, 109171. [Google Scholar] [CrossRef] [PubMed]
- Jayatunge, N.; Duncan, T.; Knapp, S.; Oligbo, N.; Thirunavukkarasu, N.; Mazibrada, J. Different clinocopathological presentations of steroid cell tumour—Report of three rare cases. Int. J. Surg. Case Rep. 2023, 102, 107842. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Hatakeyama, S.; Yoneyama. Humoral response after SARS-CoV-2 mRNA vaccination in patients with prostate cancer using steroids. Urol. Oncol. 2022, 40, 451.e1–451.e8. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Hanh, T.T.H.; Quang, T.H.; Cuong, N.X.; Nam, N.H.; Thao, D.T.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Polyhydroxylated steroids from the Vietnamese soft coral Sarcophyton ehrenbergi. Steroids 2021, 176, 108932. [Google Scholar] [CrossRef] [PubMed]




| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | - | 40.8 | 1′ | 4.54 (d, J = 7.5 Hz) | 101.0 |
| 2 | 1.59 (ddd, J = 12.3, 4.3, 2.1 Hz) 1.75 (t, J = 12.2 Hz) | 44.4 | 2′ | 3.36 (m) | 83.7 |
| 3 | 4.21 (tt, J = 11.6, 4.3 Hz) | 74.2 | 3′ | 3.54 (m) | 77.8 |
| 4 | 1.87 (m) 1.96 (ddd, J = 13.3, 4.3, 2.2 Hz) | 42.3 | 4′ | 3.33 (m) | 71.4 |
| 5 | - | 77.8 | 5′ | 3.28 (m) | 78.6 |
| 6 | - | 79.1 | 6′ | 3.68 (m) 3.85 (dd, J = 12.0, 2.3 Hz) | 62.6 |
| 7 | 6.04 (dd, J = 15.8, 1.3 Hz) | 130.9 | 1″ | 4.54 (d, J = 7.5 Hz) | 105.6 |
| 8 | 5.78 (dd, J = 15.8, 6.4 Hz) | 136.2 | 2″ | 3.24 (m) | 76.2 |
| 9 | 4.33 (d, J = 6.4 Hz) | 69.6 | 3″ | 3.27 (m) | 77.9 |
| 10 | 1.26 (d, J = 6.4 Hz) | 24.1 | 4″ | 3.28 (m) | 71.5 |
| 11 | 1.21 (s) | 26.3 | 5″ | 3.37 (m) | 77.7 |
| 12 | 0.87 (s) | 27.5 | 6″ | 3.68 (m) 3.92 (dd, J = 12.1, 1.6 Hz) | 62.6 |
| 13 | 1.11 (s) | 27.1 |
| Position | 2 | 3 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | - | 132.1 | 3.74 (m) 3.44 (m) | 68.3 |
| 2 | 7.15 (d, J = 2.0 Hz) | 119.9 | 2.26 (m) | 27.6 |
| 3 | - | 146.9 | 5.34 (dt, J = 11.3, 6.9 Hz) | 125.4 |
| 4 | - | 146.7 | 5.39 (dt, J = 11.1, 6.9 Hz) | 132.9 |
| 5 | 6.76 (d, J = 8.1 Hz) | 116.4 | 2.00 (m, J = 7.3 Hz) | 20.3 |
| 6 | 6.80 (dd, J = 2.0, 8.1 Hz) | 125.6 | 0.91 (t, J = 7.5 Hz) | 14.3 |
| 7 | 2.72 (t, J = 7.1 Hz) | 39.5 | - | - |
| 8 | 3.69 (t, J = 7.0 Hz) | 64.3 | - | - |
| 1′ | 4.78 (d, J = 7.8 Hz) | 103.6 | 4.29 (d, J = 7.7 Hz) | 101.4 |
| 2′ | 3.75 (m) | 83.3 | 3.20 (t, J = 8.4 Hz) | 82.5 |
| 3′ | 3.40 (m) | 78.2 | 3.07 (m) | 77.1 |
| 4′ | 3.43 (m) | 71.0 | 3.10 (m) | 69.9 |
| 5′ | 3.64 (t, J = 8.9 Hz) | 77.8 | 3.35 (t, J = 8.5 Hz) | 76.1 |
| 6′ | 3.77 (m) 3.71 (m) | 62.3 | 3.62 (m) 3.50 (m) | 60.9 |
| 1″ | 4.74 (d, J = 7.8 Hz) | 105.7 | 4.37 (d, J = 8.1 Hz) | 104.2 |
| 2″ | 3.28 (dd, J = 9.2, 7.9 Hz) | 75.8 | 2.98 (t, J = 8.2 Hz) | 75.0 |
| 3″ | 3.36 (m) | 78.5 | 3.12 (m) | 76.7 |
| 4″ | 3.35 (m) | 71.3 | 3.10 (m) | 69.8 |
| 5″ | 3.74 (m) | 77.7 | 3.13 (d, J = 2.6 Hz) | 76.2 |
| 6″ | 3.93 (m) 3.70 (m) | 64.3 | 3.66 (m) 3.43 (m) | 61.0 |
| Compound | SC50 (μM) | Compound | SC50 (μM) |
|---|---|---|---|
| 1 | nd * | 16 | nc ** |
| 2 | nc ** | 17 | 75.6 ± 6.1 |
| 3 | nc ** | 18 | 127.9 ± 15.4 |
| 4 | nc ** | 19 | 48.8 ± 2.9 |
| 5 | nc ** | 20 | nc ** |
| 6 | 194.9 ± 12.6 | 21 | 50.8 ± 1.4 |
| 7 | 255.2 ± 12.6 | 22 | nc ** |
| 8 | nc ** | 23 | 36.4 ± 5.0 |
| 9 | 47.3 ± 2.3 | 24 | 50.8 ± 1.4 |
| 10 | 30.5 ± 6.5 | 25 | nc ** |
| 11 | 16.2 ± 1.6 | 26 | 284.0 ± 16.5 |
| 12 | nc ** | 27 | nc ** |
| 13 | nc ** | 28 | nc ** |
| 14 | nc ** | 29 | nc ** |
| 15 | nc ** | Ascorbic acid | 15.6 ± 0.8 |
| Compounds | IC50 (μM) | Cytotoxicity (IC50) | Compounds | IC50 (μM) | Cytotoxicity (IC50) |
|---|---|---|---|---|---|
| 1 | nd * | nd * | 2–20, 22–29 | >200 | >200 |
| 21 | 28.6 ± 3.0 | >200 | Quercetin | 11.1 ± 1.4 | >50 |
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| A549 | NCI-H292 | SiHa | MKN45 | |
| 1 | nd * | nd * | nd * | nd * |
| 4 | 17.4 ± 2.4 | 22.2 ± 1.1 | 9.7 ± 0.9 | 9.5 ±0.7 |
| 5 | 0.1> | 0.1> | 0.1> | 0.1> |
| 28 | >200 | >200 | >200 | 52.8 ± 5.3 |
| 2, 3, 6–27, 29 | >200 | >200 | >200 | >200 |
| cisplatin | 19.5 ± 6.7 | 52.8 ± 5.3 | 7.0 ± 0.7 | 7.0 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-Z.; Ma, J.-C.; Deng, P.; Ren, F.-C.; Li, N. Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities. Molecules 2023, 28, 2685. https://doi.org/10.3390/molecules28062685
Liu Z-Z, Ma J-C, Deng P, Ren F-C, Li N. Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities. Molecules. 2023; 28(6):2685. https://doi.org/10.3390/molecules28062685
Chicago/Turabian StyleLiu, Zhen-Zhen, Jun-Cheng Ma, Peng Deng, Fu-Cai Ren, and Ning Li. 2023. "Chemical Constituents of Thesium chinense Turcz and Their In Vitro Antioxidant, Anti-Inflammatory and Cytotoxic Activities" Molecules 28, no. 6: 2685. https://doi.org/10.3390/molecules28062685