Niobium Complexes Supported by Chalcogen-Bridged [OEO]-Type Bis(phenolate) Ligands (E = S, Se): Synthesis, Characterization, and Phenylacetylene Polymerization
Abstract
:1. Introduction
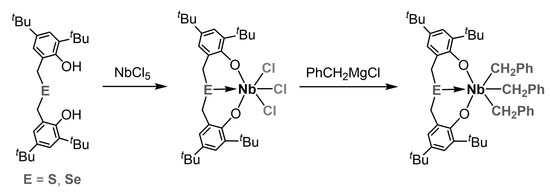
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tshuva, E.Y.; Peri, D. Modern Cytotoxic Titanium (IV) Complexes; Insights on the Enigmatic Involvement of Hydrolysis. Coord. Chem. Rev. 2009, 253, 2098–2115. [Google Scholar] [CrossRef]
- Janas, Z. Well-defined single-site thiobis(phenolate) Group 4 metal catalysts for heterogeneous olefin polymerization. Coord. Chem. Rev. 2010, 254, 2227–2233. [Google Scholar] [CrossRef]
- Jean-François Carpentier. Rare-Earth Complexes Supported by Tripodal Tetradentate Bis(phenolate) Ligands: A Privileged Class of Catalysts for Ring-Opening Polymerization of Cyclic Esters. Organometallics 2015, 34, 4175–4189.
- Tshuva, E.Y.; Versano, M.; Goldberg, I.; Kol, M.; Weitman, H.; Goldschmidt, Z. Titanium complexes of chelating dianionic amine bis(phenolate) ligands: An extra donor makes a big difference. Inorg. Chem. Commun. 1999, 2, 371–373. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Goldberg, I.; Kol, M.; Weitman, H.; Goldschmidt, Z. Novel zirconium complexes of amine bis(phenolate) ligands. Remarkable reactivity in polymerization of hex-1-ene due to an extra donor arm. Chem. Commun. 2000, 379–380. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Goldberg, I.; Kol, M.; Goldschmidt, Z. Living polymerization of 1-hexene due to an extra donor arm on a novel amine bis(phenolate) titanium catalyst. Inorg. Chem. Commun. 2000, 3, 610–614. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Goldberg, I.; Kol, M.; Goldschmidt, Z. Coordination Chemistry of Amine Bis(phenolate) Titanium Complexes: Tuning Complex Type and Structure by Ligand Modification. Inorg. Chem. 2001, 40, 4263–4270. [Google Scholar] [CrossRef]
- Chmura, A.J.; Davidson, M.G.; Jones, M.D.; Lunn, M.D.; Mahon, M.F. Group 4 complexes of amine bis(phenolate)s and their application for the ring opening polymerisation of cyclic esters. Dalton Trans. 2006, 887–889. [Google Scholar] [CrossRef]
- Liang, L.-C.; Lin, S.-T.; Chien, C.-C. Titanium Complexes of Tridentate Aminebiphenolate Ligands Containing Distinct N-Alkyls: Profound N-Substituent Effect on Ring-Opening Polymerization Catalysis. Inorg. Chem. 2013, 52, 1780–1786. [Google Scholar] [CrossRef]
- Manne, R.; Miller, M.; Duthie, A.; Guedes da Silva, M.F.C.G.; Tshuva, E.Y.; Basu Baul, T.S.B. Cytotoxic Homoleptic Ti (Iv) Compounds of ONO-Type Ligands: Synthesis, Structures and Anti-Cancer Activity. Dalton Trans. 2019, 48, 304–314. [Google Scholar] [CrossRef]
- Shpilt, Z.; Manne, R.; Rohman, M.A.; Mitra, S.; Tiekink, E.R.T.; Basu Baul, T.S.; Tshuva, E.Y. Homoleptic Ti[ONO]2 Type Complexes of Amino-acid-tethered Phenolato Schiff-base Ligands: Synthesis, Characterization, Time-resolved Fluorescence Spectroscopy, and Cytotoxicity against Ovarian and Colon Cancer Cells. Appl. Organomet. Chem. 2020, 34, e5309. [Google Scholar] [CrossRef]
- Nakayama, Y.; Watanabe, K.; Uetama, N.; Nakamura, A.; Harada, A.; Okuda, J. Titanium Complexes Having Chelating Diaryloxo Ligands Bridged by Tellurium and Their Catalytic Behavior in the Polymerization of Ethylene. Organometallics 2000, 19, 2498–2503. [Google Scholar] [CrossRef]
- Fokken, S.; Spaniol, T.P.; Kang, H.-C.; Massa, W.; Okuda, J. Titanium Complexes of Chelating Bis(phenolato) Ligands with Long Titanium−Sulfur Bonds. A Novel Type of Ancillary Ligand for Olefin Polymerization Catalysts. Organometallics 1996, 15, 5069–5072. [Google Scholar] [CrossRef]
- Sernetz, F.G.; Mülhaupt, R.; Fokken, S.; Okuda, J. Copolymerization of Ethene with Styrene Using Methylaluminoxane-Activated Bis(phenolate) Complexes. Macromolecules 1997, 30, 1562–1569. [Google Scholar] [CrossRef]
- Natrajan, L.S.; Wilson, C.; Okuda, J.; Arnold, P.L. Group 4 Complexes of Chelating Dianionic [OSO] Binaphtholate Ligands; Synthesis and Alkene Polymerisation Catalysis. Eur. J. Inorg. Chem. 2004, 2004, 3724–3732. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Lyadov, A.S.; Kurmaev, D.A.; Zubkevich, S.V.; Shatokhin, S.S.; Simikin, V.E.; Mikhailik, E.S.; Golubev, E.K.; Nikiforova, G.G.; et al. A Titanium(IV) Complex with an OSO-Type Ligand as a Catalyst for the Synthesis of Ultrahigh-Molecular-Weight Polyethylene. Pet. Chem. 2020, 60, 329–333. [Google Scholar] [CrossRef]
- Miyatake, T.; Mizunuma, K.; Seki, Y.; Kakugo, M. 2,2′-thiobis(6-tert-butyl-4-methylphenoxy)titanium or zirconium complex-methylalumoxane catalysts for polymerization of olefins. Makromol. Chem. Rapid Commun. 1989, 10, 349–352. [Google Scholar] [CrossRef]
- Kakugo, M.; Miyatake, T.; Mizunuma, K. Polymerization of styrene and copolymerization of styrene with olefin in the presence of soluble Ziegler-Natta catalysts. In Studies in Surface Science and Catalysis; Elsevier: Tokyo, Japan, 1990; Volume 56, pp. 517–529. ISBN 978-0-444-98747-1. [Google Scholar]
- Janas, Z.; Jerzykiewicz, L.B.; Przybylak, K.; Sobota, P.; Szczegot, K. Titanium Complexes Stabilized by a Sulfur-Bridged Chelating Bis(aryloxo) Ligand as Active Catalysts for Olefin Polymerization. Eur. J. Inorg. Chem. 2004, 2004, 1639–1645. [Google Scholar] [CrossRef]
- Janas, Z.; Jerzykiewicz, L.B.; Sobota, P.; Szczegot, K.; Wioeniewska, D. Homo- and Heterometallic Aluminum and Titanium Complexes of Tridentate (OSO) Ligand: Synthesis, Structure, and Catalytic Activity. Organometallics 2005, 24, 3987–3994. [Google Scholar] [CrossRef]
- Janas, Z.; Jerzykiewicz, L.B.; Przybylak, K.; Sobota, P.; Szczegot, K.; Wioeniewska, D. Titanium Complexes of Chelating, Dianionic O,S,O-Bisphenolato Ligands: Syntheses, Characterisation, and Catalytic Activity. Eur. J. Inorg. Chem. 2005, 2005, 1063–1070. [Google Scholar] [CrossRef]
- Wisniewska, D.; Janas, Z.; Sobota, P.; Jerzykiewicz, L.B. Dimethyl Group IV Metal Complexes of the OSO-Type Ligand Bearing AlMe2 Moieties. Organometallics 2006, 25, 6166–6169. [Google Scholar]
- Agapie, T.; Henling, L.M.; Dipasquale, A.G.; Rheingold, A.L.; Bercaw, J.E. Zirconium and Titanium Complexes Supported by Tridentate LX2 Ligands Having Two Phenolates Linked to Furan, Thiophene, and Pyridine Donors: Precatalysts for Propylene Polymerization and Oligomerization. Organometallics 2008, 27, 6245–6256. [Google Scholar]
- Kirillov, E.; Roisnel, T.; Razavi, A.; Carpentier, J.F. Group 4 Post-metallocene Complexes Incorporating Tridentate Silyl-Substituted Bis(naphthoxy)pyridine and Bis(naphthoxy)thiophene Ligands: Probing Systems for “Oscillating” Olefin Polymerization Catalysis. Organometallics 2009, 28, 5036–5051. [Google Scholar]
- Song, T.; He, J.; Liang, L.; Liu, N.; Li, F.; Tong, X.; Mu, X.; Mu, Y. Titanium and zirconium complexes bearing new tridentate [OSO] bisphenolato-based ligands: Synthesis, characterization and catalytic properties for alkene polymerization. Dalton Trans. 2019, 48, 13719–13731. [Google Scholar]
- Redshaw, C.; Homden, D.M.; Rowan, M.A.; Elsegood, M.R.J. Niobium-based ethylene polymerization procatalysts bearing di- and triphenolate ligands. Inorg. Chim. Acta. 2005, 358, 4067–4074. [Google Scholar]
- Nakata, N.; Toda, T.; Ishii, A. Recent advances in the chemistry of Group 4 metal complexes incorporating [OSSO]-type bis(phenolato) ligands as post-metallocene catalysts. Polym. Chem. 2011, 2, 1597–1610. [Google Scholar]
- Ishii, A.; Toda, T.; Nakata, N.; Matsuo, T. Zirconium Complex of an [OSSO]-Type Diphenolate Ligand Bearingtrans-1,2-Cyclooctanediylbis(thio) Core: Synthesis, Structure, and Isospecific 1-Hexene Polymerization. J. Am. Chem. Soc. 2009, 131, 13566–13567. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Titanium Complexes Supported by an [OSSO]-Type Bis(phenolato) Ligand Based on a trans-Cyclooctanediyl Platform: Synthesis, Structures, and 1-Hexene Polymerization. Inorg. Chem. 2012, 51, 274–281. [Google Scholar] [PubMed]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled Isospecific Polymerization of α-Olefins by Hafnium Complex Incorporating with a trans-Cyclooctanediyl-Bridged [OSSO]-Type Bis(phenolate) Ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar]
- Toda, T.; Nakata, N.; Matsuo, T.; Ishii, A. Synthesis, Structure, and 1-Hexene Polymerization Catalytic Ability of Group 5 Metal Complexes Incorporating an [OSSO]-Type Ligand. ACS Catal. 2013, 3, 1764–1767. [Google Scholar] [CrossRef]
- Saito, Y.; Nakata, N.; Ishii, A. Highly Isospecific Polymerization of Silyl-protected ω-Alkenols with an [OSSO]-Type Bis(phenolato) Dichloro Zirconium(IV) Precatalyst. Macromol. Rapid Commun. 2016, 37, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Nakata, N.; Toda, T.; Saito, Y.; Watanabe, T.; Ishii, A. Highly Active and Isospecific Styrene Polymerization Catalyzed by Zirconium Complexes Bearing Aryl-substituted [OSSO]-Type Bis(phenolate) Ligands. Polymers 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, A.; Ikuma, K.; Nakata, N.; Nakamura, K.; Kuribayashi, H.; Takaoki, K. Zirconium and Hafnium Complexes with Cycloheptane- or Cyclononane-Fused [OSSO]-Type Bis(phenolato) Ligands: Synthesis, Structure, and Highly Active 1-Hexene Polymerization and Ring-Size Effects of Fused Cycloalkanes on the Activity. Organometallics 2017, 36, 3954–3966. [Google Scholar] [CrossRef]
- Jura, M.; Levason, W.; Ratnani, R.; Reid, G.; Webster, M. Six- and eight-coordinate thio- and seleno-ether complexes of NbF5 and some comparisons with NbCl5 and NbBr5 adducts. Dalton Trans. 2010, 39, 883–891. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Pampaloni, G. Niobium complexes as catalytic precursors for the polymerization of olefins. Coord. Chem. Rev. 2010, 254, 525–536. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Hector, A.L.; Light, M.E.; Reid, G. Niobium tetrachloride complexes with thio-, seleno- and telluro-ether coordination—Synthesis and structures. Dalton Trans. 2016, 45, 16262–16274. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-P.; Hector, A.L.; Levason, W.; Reid, G. Chalcogenoether complexes of Nb(V) thio- and seleno-halides as single source precursors for low pressure chemical vapour deposition of NbS2 and NbSe2 thin films. Dalton Trans. 2017, 46, 9824–9832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Kawaguchi, H. Tridentate Aryloxide Ligands: New Supporting Ligands in Coordination Chemistry of Early Transition Metals. Chem. Lett. 2004, 33, 640–645. [Google Scholar] [CrossRef]
- Akagi, F.; Ishida, Y.; Matsuo, T.; Kawaguchi, H. Synthesis and Reactivity of Niobium Complexes Having a Tripodal Triaryloxide Ligand in Bidentate, Tridentate, and Tetradentate Coordination Modes. Dalton Trans. 2011, 40, 2375–2382. [Google Scholar] [CrossRef]
- Simionescu, C.I.; Percec, V.; Dumitrescu, S. Polymerization of acetylenic derivatives XXX. Isomers of polyphenylacetylene. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 2497–2509. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision B. 04; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]






| Bond Lengths | [Å] | Bond Angles | [°] |
|---|---|---|---|
| Nb1–O1 | 1.888(5) | Se1–Nb1–Cl1 | 177.56(6) |
| Nb1–O2 | 1.878(5) | O1–Nb1–Cl2 | 163.18(16) |
| Nb1–Cl1 | 2.326(2) | O2–Nb1–Cl3 | 161.35(17) |
| Nb1–Cl2 | 2.385(2) | Se1–Nb1–O1 | 81.68(15) |
| Nb1–Cl3 | 2.387(2) | Se1–Nb1–O2 | 82.16(16) |
| Nb1–Se1 | 2.7075(11) |
| Bond Lengths | [Å] | Bond Angles | [°] |
|---|---|---|---|
| Nb1–O1 | 1.901(2) | Se1–Nb1–C38 | 157.40(7) |
| Nb1–O2 | 1.915(2) | O1–Nb1–O2 | 154.05(8) |
| Nb1–C31 | 2.212(3) | C31–Nb1–C45 | 145.11(11) |
| Nb1–C38 | 2.346(3) | Se1–Nb1–O1 | 77.37(6) |
| Nb1–C45 | 2.275(3) | Se1–Nb1–O2 | 78.76(6) |
| Nb1–Se1 | 2.8940(7) | C32–C31–Nb1 | 98.62(17) |
| C39–C38–Nb1 | 128.4(2) | ||
| Nb1...C32 | 2.844 | C46–C45–Nb1 | 114.2(2) |
| Entry | Cat. | Temp. [°C] | Yield of PPA [mg] 3 | Mw [g mol−1] 4 | PDI [Mw/Mn] 4 | Yield of Oligomer [mg] 3 | Yield of TPBs [mg] 3 | Ratio of TPBs [1,3,5-/1,2,4-] 5 |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 60 | 11 | 10,000 | 1.8 | 26 | 4 | 3.0/1.0 |
| 2 | 5 | 80 | 78 | 24,000 | 2.5 | 50 | 78 | 0.9/1.0 |
| 3 2 | 5 | 60 | 80 | 25,000 | 2.5 | 31 | 51 | 1.0/1.0 |
| 4 | 6 | 60 | 141 | 86,000 | 2.4 | 31 | 30 | 1.1/1.0 |
| 5 | 6 | 80 | 218 | 44,000 | 2.8 | 42 | 90 | 0.5/1.0 |
| 6 2 | 6 | 60 | 266 | 49,000 | 2.3 | 25 | 84 | 1.3/1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Ishii, A.; Nakata, N. Niobium Complexes Supported by Chalcogen-Bridged [OEO]-Type Bis(phenolate) Ligands (E = S, Se): Synthesis, Characterization, and Phenylacetylene Polymerization. Molecules 2023, 28, 2573. https://doi.org/10.3390/molecules28062573
An J, Ishii A, Nakata N. Niobium Complexes Supported by Chalcogen-Bridged [OEO]-Type Bis(phenolate) Ligands (E = S, Se): Synthesis, Characterization, and Phenylacetylene Polymerization. Molecules. 2023; 28(6):2573. https://doi.org/10.3390/molecules28062573
Chicago/Turabian StyleAn, Jing, Akihiko Ishii, and Norio Nakata. 2023. "Niobium Complexes Supported by Chalcogen-Bridged [OEO]-Type Bis(phenolate) Ligands (E = S, Se): Synthesis, Characterization, and Phenylacetylene Polymerization" Molecules 28, no. 6: 2573. https://doi.org/10.3390/molecules28062573