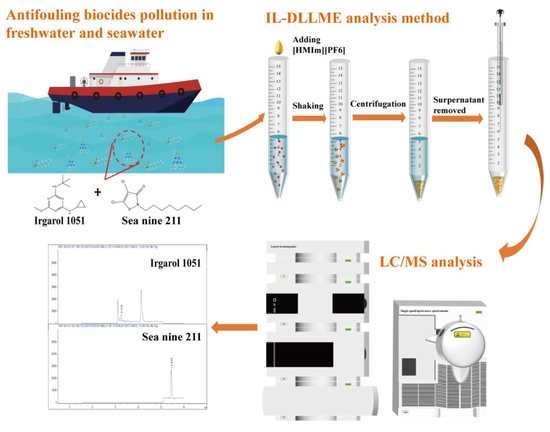
Ionic Liquid-Dispersive Micro-Extraction and Detection by High Performance Liquid Chromatography–Mass Spectrometry for Antifouling Biocides in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of IL-DLLME Procedure
2.1.1. Effect of Amount of IL
2.1.2. Selection of Disperser Solvent and Effect of Volume
2.1.3. Salt Effect
2.1.4. Sample pH
2.1.5. Effect of Cooling Temperature
2.2. Method Validation
2.3. Real Water Samples Analysis
2.4. Comparison of IL-DLLME with Other Sample Preparation Techniques
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Apparatus
3.3. IL-DLLME Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Liu, D.; Pacepavicius, G.J.; Maguire, R.J.; Lau, Y.L.; Okamura, H.; Aoyama, I. Survey for the occurrence of the new antifouling compound Irgarol 1051 in the aquatic environment. Water Res. 1999, 33, 2833–2843. [Google Scholar] [CrossRef]
- Scarlett, A.; Donkin, P.; Fileman, T.W.; Morris, R.J. Occurrence of the antifouling herbicide, irgarol 1051, within coastal-water seagrasses from Queensland, Australia. Mar. Pollut. Bull. 1999, 38, 687–691. [Google Scholar] [CrossRef]
- Ishibashi, H.; Takaichi, D.; Takeuchi, I. Effects of the herbicide Irgarol 1051 on the transcriptome of hermatypic coral Acropora tenuis and its symbiotic dinoflagellates. Sci. Total Environ. 2021, 780, 146542. [Google Scholar] [CrossRef]
- US EPA. Irgarol. Interim Registration Review Decision. Case Number 5031. Docket Number EPA-HQ-OPP-2010-0003. 2021. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2010-0003-0017 (accessed on 9 December 2022).
- EU Commission. Commission Implementing Decision (EU) 2016/107 of 27 January 2016 not Approving Cybutryne as an Existing Active Substance for Use in Biocidal Products for Product-Type 21. 2016. Available online: https://www.legislation.gov.uk/eudn/2016/107/2020-12-31 (accessed on 9 December 2022).
- Okamura, H.; Watanabe, T.; Aoyama, I.; Hasobe, M. Toxicity evaluation of new antifouling compounds using suspension-cultured fish. Chemosphere 2002, 46, 945–951. [Google Scholar] [CrossRef]
- Kobayashi, N.; Okamura, H. Effects of new antifouling compounds on the development of sea urchin. Mar. Pollut. Bull. 2002, 44, 748–751. [Google Scholar] [CrossRef]
- de Campos, B.G.; Figueiredo, J.; Perina, F.; Abessa, D.M.d.S.; Loureiro, S.; Martins, R. Occurrence, effects and environmental risk of antifouling biocides (EU PT21): Are marine ecosystems threatened? Crit. Rev. Environ. Sci. Technol. 2022, 52, 3179–3210. [Google Scholar] [CrossRef]
- Lam, N.H.; Jeong, H.-h.; Kang, S.-d.; Kim, D.-J.; Ju, M.-J.; Horiguchi, T.; Cho, H.-S. Organotins and new antifouling biocides in water and sediments from three Korean Special Management Sea Areas following ten years of tributyltin regulation: Contamination profiles and risk assessment. Mar. Pollut. Bull. 2017, 121, 302–312. [Google Scholar] [CrossRef]
- Diniz, L.G.R.; Jesus, M.S.; Dominguez, L.A.E.; Fillmann, G.; Vieira, E.M.; Franco, T. First appraisal of water contamination by antifouling booster biocide of 3rd generation at Itaqui Harbor (São Luiz-Maranhão-Brazil). J. Braz. Chem. Soc. 2014, 25, 380–388. [Google Scholar] [CrossRef]
- Mochida, K.; Hano, T.; Onduka, T.; Ichihashi, H.; Amano, H.; Ito, M.; Ito, K.; Tanaka, H.; Fujii, K. Spatial analysis of 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (Sea-Nine 211) concentrations and probabilistic risk to marine organisms in Hiroshima Bay, Japan. Environ. Pollut. 2015, 204, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.; Ferrer, I.; Barceló, D. Part-per-trillion level determination of antifouling pesticides and their byproducts in seawater samples by off-line solid-phase extraction followed by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2000, 879, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Basheer, C.; Tan, K.S.; Lee, H.K. Organotin and Irgarol-1051 contamination in Singapore coastal waters. Mar. Pollut. Bull. 2002, 44, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, Á.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Applicability of microwave-assisted extraction combined with LC–MS/MS in the evaluation of booster biocide levels in harbour sediments. Chemosphere 2011, 82, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bester, K.; Lamani, X. Determination of biocides as well as some biocide metabolites from facade run-off waters by solid phase extraction and high performance liquid chromatographic separation and tandem mass spectrometry detection. J. Chromatogr. A 2010, 1217, 5204–5214. [Google Scholar] [CrossRef]
- Lambropoulou, D.A.; Sakkas, V.A.; Albanis, T.A. Analysis of antifouling biocides Irgarol 1051 and Sea Nine 211 in environmental water samples using solid-phase microextraction and gas chromatography. J. Chromatogr. A 2002, 952, 215–227. [Google Scholar] [CrossRef]
- Giraldez, I.; Chaguaceda, E.; Bujalance, M.; Morales, E. Determination of five booster biocides in seawater by stir bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1271, 17–26. [Google Scholar] [CrossRef]
- Saleh, A.; Fumani, N.S.; Molaei, S. Microfunnel-supported liquid-phase microextraction: Application to extraction and determination of Irgarol 1051 and diuron in the Persian Gulf seawater samples. J. Chromatogr. A 2014, 1356, 32–37. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. TrAC Trends Anal. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Lai, X.; Ruan, C.; Liu, R.; Liu, C. Application of ionic liquid-based dispersive liquid–liquid microextraction for the analysis of ochratoxin A in rice wines. Food Chem. 2014, 161, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M. Dispersive liquid-liquid microextraction: Evolution in design, application areas, and green aspects. TrAC Trends Anal. Chem. 2022, 152, 116636. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Wang, K.; Liang, Q.; Xue, X.-F.; Zhao, L.-W.; Chen, D.-F.; Wu, L.-M.; Guo, R.; Xiong, C.-L. Ionic liquid dispersive liquid–liquid microextraction for pesticide residue analysis in honey. J. Apic. Res. 2020, 59, 458–467. [Google Scholar] [CrossRef]
- Ullah, H.; Wilfred, C.D.; Shaharun, M.S. Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Sep. Sci. Technol. 2019, 54, 559–579. [Google Scholar] [CrossRef]
- Feng, J.; Loussala, H.M.; Han, S.; Ji, X.; Li, C.; Sun, M. Recent advances of ionic liquids in sample preparation. TrAC Trends Anal. Chem. 2020, 125, 115833. [Google Scholar] [CrossRef]
- Lim, J.R.; Chua, L.S.; Mustaffa, A.A. Ionic liquids as green solvent and their applications in bioactive compounds extraction from plants. Process Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Zhou, G.-S.; Yuan, Y.-C.; Yin, Y.; Tang, Y.-P.; Xu, R.-J.; Liu, Y.; Chen, P.-D.; Yin, L.; Duan, J.-A. Hydrophilic interaction chromatography combined with ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction for determination of underivatized neurotransmitters in dementia patients’ urine samples. Anal. Chim. Acta 2020, 1107, 74–84. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Shi, Y.-P. Temperature-assisted ionic liquid dispersive liquid–liquid microextraction combined with high performance liquid chromatography for the determination of anthraquinones in Radix et Rhizoma Rhei samples. Talanta 2010, 82, 1010–1016. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Jiang, X. Determination of phthalate esters in water samples by ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction coupled with high-performance liquid chromatography. Anal. Chim. Acta 2011, 689, 137–142. [Google Scholar] [CrossRef]
- Unsal, Y.E.; Soylak, M.; Tuzen, M. Ultrasound-assisted ionic liquid-based dispersive liquid–liquid microextraction for preconcentration of patent blue V and its determination in food samples by UV–visible spectrophotometry. Environ. Monit. Assess. 2015, 187, 203. [Google Scholar] [CrossRef]
- Elik, A.; Demirbas, A.; Altunay, N. Analysis of Zinc and Chromium in grain samples using ionic liquid-based ultrasound-assisted microextraction followed by flame-AAS after microwave digestion. Biol. Trace Elem. Res. 2020, 198, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Adhami, K.; Asadollahzadeh, H.; Ghazizadeh, M. Preconcentration and determination of nickel (II) and copper (II) ions, in vegetable oils by [TBP] [PO4] IL-based dispersive liquid–liquid microextraction technique, and flame atomic absorption spectrophotometry. J. Food Compos. Anal. 2020, 89, 103457. [Google Scholar] [CrossRef]
- Yang, J.; Fan, C.; Tang, G.; Zhang, W.; Dong, H.; Liang, Y.; Wang, Y.; Zou, M.; Cao, Y. Relationship between the structure of ionic liquid and its enrichment ability to trace fungicides from an environmental water sample. J. Agric. Food. Chem. 2018, 66, 9418–9425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tan, Z.; Zhao, J.; Wen, Y.; Fan, S.; Liu, C. Determination of pyrethroid residues in herbal tea using temperature-controlled ionic liquid dispersive liquid-liquid microextraction by high performance liquid chromatography. Sci. Rep. 2020, 10, 4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Liu, X.; Yang, X.; Wang, Y.; Zhao, L. Ionic liquid-based dispersive liquid–liquid microextraction followed by magnetic solid-phase extraction for determination of quinolones. Microchim. Acta 2021, 189, 8. [Google Scholar] [CrossRef]
- Gao, S.; Jin, H.; You, J.; Ding, Y.; Zhang, N.; Wang, Y.; Ren, R.; Zhang, R.; Zhang, H. Ionic liquid-based homogeneous liquid–liquid microextraction for the determination of antibiotics in milk by high-performance liquid chromatography. J. Chromatogr. A 2011, 1218, 7254–7263. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, R.; Zhang, X.; Zhang, D. Ionic liquid–based dispersive liquid–liquid micro-extraction of five organophosphorus pesticides in coarse cereals. Food Anal. Methods 2021, 14, 10–17. [Google Scholar] [CrossRef]
- Taziki, M.; Shemirani, F.; Majidi, B. Robust ionic liquid–based dispersive liquid–liquid microextraction method for determination of chromium(vi) in saline solutions. Commun. Soil Sci. Plant Anal. 2013, 44, 3400–3411. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Chen, D.; Zhou, Y.; Lin, Q.; Ma, X.; Zhang, M.; Hu, H. [Hmim]PF6 enhanced the extraction of polycyclic aromatic hydrocarbons from soil with the QuEChERS method. Arabian J. Chem. 2020, 13, 4102–4110. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Yang, S.; Liu, F. Ionic liquid-based dispersive liquid–liquid microextraction following high-performance liquid chromatography for the determination of fungicides in fruit juices. Food Anal. Methods 2013, 6, 481–487. [Google Scholar] [CrossRef]
- Marube, L.C.; Caldas, S.S.; dos Santos, E.O.; Michaelsen, A.; Primel, E.G. Multi-residue method for determination of thirty-five pesticides, pharmaceuticals and personal care products in water using ionic liquid-dispersive liquid-liquid microextraction combined with liquid chromatography-tandem mass spectrometry. J. Braz. Chem. Soc. 2018, 29, 1349–1359. [Google Scholar] [CrossRef]
- Kong, L.; Wang, J.; Gao, Q.; Li, X.; Zhang, W.; Wang, P.; Ma, L.; He, L. Simultaneous determination of fat-soluble vitamins and carotenoids in human serum using a nanostructured ionic liquid based microextraction method. J. Chromatogr. A 2022, 1666, 462861. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhu, Y.; Yang, Y. Dispersive liquid–liquid microextraction followed by magnetic solid-phase extraction for determination of four parabens in beverage samples by ultra-performance liquid chromatography tandem mass spectrometry. Food Anal. Methods 2018, 11, 797–807. [Google Scholar] [CrossRef]
- Lubomirsky, E.; Padró, J.M.; Reta, M.R. Development of a dispersive liquid-liquid microextraction technique for the analysis of aryloxyphenoxy-propionate herbicides in soy-based foods. Microchem. J. 2016, 129, 63–70. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, H.; Peng, B.; Li, S.; Zhou, Z. Comparison of the performance of conventional, temperature-controlled, and ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction combined with high-performance liquid chromatography in analyzing pyrethroid pesticides in honey samples. J. Chromatogr. A 2011, 1218, 6621–6629. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Alonso, D.J.; Garza-Tapia, M.; Chávez-Montes, A.; González-Horta, A.; Waksman de Torres, N.H.; Castro-Ríos, R. New temperature-assisted ionic liquid-based dispersive liquid–liquid microextraction method for the determination of glyphosate and aminomethylphosphonic acid in water samples. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 147–155. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE/11312/2021. 2021. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 10 January 2023).
- van Wezel, A.P.; van Vlaardingen, P. Environmental risk limits for antifouling substances. Aquat. Toxicol. 2004, 66, 427–444. [Google Scholar] [CrossRef]
- European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013L0039 (accessed on 7 January 2023).
- Agüera, A.; Piedra, L.; Hernando, M.a.D.; Fernández-Alba, A.R. Multiresidue method for the analysis of five antifouling agents in marine and coastal waters by gas chromatography–mass spectrometry with large-volume injection. J. Chromatogr. A 2000, 889, 261–269. [Google Scholar] [CrossRef]
- Lamoree, M.H.; Swart, C.P.; van der Horst, A.; van Hattum, B. Determination of diuron and the antifouling paint biocide Irgarol 1051 in Dutch marinas and coastal waters. J. Chromatogr. A 2002, 970, 183–190. [Google Scholar] [CrossRef]
- Peñalver, A.; Pocurull, E.; Borrull, F.; Marcé, R.M. Solid-phase microextraction of the antifouling Irgarol 1051 and the fungicides dichlofluanid and 4-chloro-3-methylphenol in water samples. J. Chromatogr. A 1999, 839, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kock-Schulmeyer, M.; Postigo, C.; Farre, M.; Barcelo, D.; de Alda, M.L. Medium to highly polar pesticides in seawater: Analysis and fate in coastal areas of Catalonia (NE Spain). Chemosphere 2019, 215, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Batista-Andrade, J.A.; Caldas, S.S.; Arias, J.L.D.; Castro, I.B.; Fillmann, G.; Primel, E.G. Antifouling booster biocides in coastal waters of Panama: First appraisal in one of the busiest shipping zones. Mar. Pollut. Bull. 2016, 112, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Du, J.; Dong, X.B.; Huang, Y.; Xie, H.J.; Chen, J.W.; Li, X.H.; Kadokami, K. Occurrence and ecological risks of 156 pharmaceuticals and 296 pesticides in seawater from mariculture areas of Northeast China. Sci. Total Environ. 2021, 792, 148375. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Gardinali, P.R. Risk assessment of triazine herbicides in surface waters and bioaccumulation of Irgarol and M1 by submerged aquatic vegetation in Southeast Florida. Sci. Total Environ. 2016, 541, 1556–1571. [Google Scholar] [CrossRef]
- Ali, H.R.; Ariffin, M.M.; Omar, T.F.T.; Ghazali, A.; Sheikh, M.A.; Shazili, N.A.M.; Bachok, Z. Antifouling paint biocides (Irgarol 1051 and Diuron) in the selected ports of Peninsular Malaysia: Occurrence, seasonal variation, and ecological risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 52247–52257. [Google Scholar] [CrossRef]
- Ansanelli, G.; Manzo, S.; Parrella, L.; Massanisso, P.; Chiavarini, S.; Di Landa, G.; Ubaldi, C.; Cannarsa, S.; Cremisini, C. Antifouling biocides (Irgarol, Diuron and dichlofluanid) along the Italian Tyrrhenian coast: Temporal, seasonal and spatial threats. Reg. Stud. Mar. Sci. 2017, 16, 254–266. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Lee, Y.W. Determination of five alternative antifouling agents found along the Korean coasts. Water Environ. Res 2017, 89, 622–628. [Google Scholar] [CrossRef]






| Sample | Deionized Water | Lake Water | Seawater | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irgarol 1051 | Spiked level (μg L−1) | 0.02 | 0.1 | 1.0 | 0.02 | 0.1 | 1.0 | 0.1 | 1.0 | 5.0 |
| Recovery (%) | 87.0 | 80.3 | 85.7 | 85.7 | 90.2 | 81.0 | 79.1 | 82.4 | 85.2 | |
| RSD (%) | 5.4 | 1.2 | 2.5 | 3.9 | 4.2 | 5.6 | 5.6 | 1.2 | 3.1 | |
| LOQ, LOD (μg L−1) | 0.02, 0.01 | 0.02, 0.01 | 0.1, 0.05 | |||||||
| Sea-Nine 211 | Spiked level (μg L−1) | 0.1 | 1.0 | 5.0 | 0.1 | 0.5 | 5.0 | 0.5 | 5.0 | 10.0 |
| Recovery (%) | 86.9 | 90.3 | 84.7 | 84.4 | 83.3 | 80.6 | 78.7 | 86.1 | 89.3 | |
| RSD (%) | 7.5 | 4.5 | 2.7 | 5.0 | 4.1 | 3.1 | 1.7 | 6.7 | 4.6 | |
| LOQ, LOD (μg L−1) | 0.06, 0.02 | 0.06, 0.02 | 0.5, 0.1 | |||||||
| Method | Sample Amount (mL) | Extraction Solvent | Solvent Volume a (mL) | Extraction Time b (min) | Extraction Recovery (%) | LOD (μg L−1) | RSD% |
|---|---|---|---|---|---|---|---|
| SPE-GC-MS [53] | 200 | EA | 15 | 46 | 42–95 | 0.0012–0.0015 | <10 |
| SPE-LC-MS/MS [54] | 100 | ACN | 12 | Not given | 77–93 | 0.002 | <8 |
| SPME-GC-MS [55] | 3 | — | — | 60 | Not given | 0.05–0.2 | <20 |
| SPE-LC-MS/MS [56] | 1000 | MeOH, DCM | 9 | 200 | 80–120 | 0.001 | <18 |
| SPE-LC-MS/MS [57] | 250 | MeOH, DCM | 8 | 25 | 78–120 | 0.0003–0.0027 | <13 |
| SPE-LC-QTOF/MS [58] | 200 | MeOH, DCM | 8 | 60 | 79.7–119.2 | Not given | 17.7–27.7 |
| LLE-GC-MS [59] | 2000 | DCM | 50 | Not given | 70–120 | 0.001 | 30 |
| SPE-GC-MS [60] | 2000 | EA, AC | 15 | 145 | >90 | 0.001 | <10 |
| SPE-LC-MS [61] | 500 | 10 mM HAc MeOH | 15 | 65 | 82.5–111 | 0.0002–0.001 | 3–5 |
| SBSE-TD-GC-MS [20] | 10 | — | — | 90 | 72–125 | 0.005–0.9 | 7–15 |
| LLE-GC-MS [62] | 1000 | Toluene | 1 | 60 | 73.55–120.28 | 0.00177–0.01242 | 1.64–4.87 |
| MF-LPME-HPLC-UV [21] | 300 | Toluene | 0.4 | 90 | Not given | 0.001–0.0048 | <12 |
| IL-DLLME method | 5 | [HMIm][PF6] | 0.046 | 1 | 80–90 | 0.01–0.1 | <8 |
| Analyte | Chemical Structure | Molecular Weight | Retention Time (min) | Mass Ions (m/z) | Fragmentor Voltage (V) |
|---|---|---|---|---|---|
| Irgarol 1051 |  | 253.1 | 8.53 | 254.0 [M + H]+ 198.0 * | 120 230 |
| Sea-Nine 211 |  | 281.0 | 12.89 | 282.0 [M + H]+ 304.0 * [M + Na]+ | 90 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wu, T.; Yu, C.; Liu, S.; Pan, C. Ionic Liquid-Dispersive Micro-Extraction and Detection by High Performance Liquid Chromatography–Mass Spectrometry for Antifouling Biocides in Water. Molecules 2023, 28, 1263. https://doi.org/10.3390/molecules28031263
Zhou L, Wu T, Yu C, Liu S, Pan C. Ionic Liquid-Dispersive Micro-Extraction and Detection by High Performance Liquid Chromatography–Mass Spectrometry for Antifouling Biocides in Water. Molecules. 2023; 28(3):1263. https://doi.org/10.3390/molecules28031263
Chicago/Turabian StyleZhou, Li, Tong Wu, Chuanshan Yu, Shaowen Liu, and Canping Pan. 2023. "Ionic Liquid-Dispersive Micro-Extraction and Detection by High Performance Liquid Chromatography–Mass Spectrometry for Antifouling Biocides in Water" Molecules 28, no. 3: 1263. https://doi.org/10.3390/molecules28031263