Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life
Abstract
:1. Introduction
2. Results
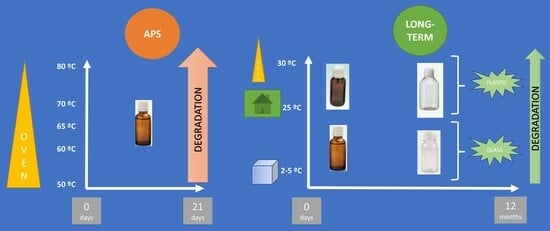
2.1. APS Study
2.2. Long-Term Study
2.3. Container Permeability Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Accelerated Predictive Stability (APS) Studies
4.2.2. Long-Term Stability Study
4.2.3. APS Modeling
4.2.4. Permeability Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ICH. Q1A(R2): Stability testing of new drug substances and products. In ICH Harmonised Tripartite Guideline Stability; ICH: Geneva, Switzerland, 2003; pp. 1–18. [Google Scholar]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C. The application of the Accelerated Stability Assessment Program (ASAP) to quality by design (QbD) for drug product stability. AAPS PharmSciTech 2011, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Rack, C. An Introduction to the Accelerated Stability Assesment Program (ASAP). Am. Pharm. Rev. 2017, 20, 86–90. [Google Scholar]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Scrivens, G.; Clancy, D.; Gerst, P. Theory and Fundamentals of Accelerated Predictive Stability (APS) studies. In Accelerated Predictive Stability (APS) Fundamentals and Pharmaceutical Industry Practices; Academic Press: Cambridge, MA, USA, 2018; pp. 33–72. [Google Scholar]
- USP. Stability Considerations in Dispensing Practice (1191); United States Pharmacopeia (USP): North Bethesda, MD, USA, 2020. [Google Scholar]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef] [PubMed]
- González-González, O.; Leal, E.; Martín-Martínez, M.; Bautista, L.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Guiding Clinical Prescription of Topical Extemporaneous Formulations of Sodium Cromoglycate Based on Pharmaceutical Performance. Pharmaceutics 2023, 15, 1609. [Google Scholar] [CrossRef] [PubMed]
- Purdy, K.R. Aspect of Chlorhexidine Degradation. Ph.D. Thesis, University of Bath, Bath, UK, 1987. [Google Scholar]
- Zong, Z.; Kirsch, L.E. Studies on the Instability of Chlorhexidine, Part I: Kinetics and Mechanisms. J. Pharm. Sci. 2012, 101, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.A.; Sousa, C.P.; Morais, S.; de Lima-Neto, P.; Correia, A.N.; Homem-De-Mello, P. Evaluation of degradation mechanism of chlorhexidine by means of Density Functional Theory calculations. Comput. Biol. Chem. 2017, 71, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Alam, M.S.; Alam, N.; Anwer, T.; Safhi, M.M.A. Accelerated Stability Testing of a Clobetasol Propionate-Loaded Nanoemulsion as per ICH Guidelines. Sci. Pharm. 2013, 81, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Medicamentos y Productos Sanitarios. FN/2003/PO/015 Solución acuosa al 0.1% de Clorhexidina. In Formulario Nacional; Agencia Española de Medicamentos y Productos Sanitarios: Madrid, Spain, 2003. [Google Scholar]
- Onetto, D.; Correa, V.; Araya, P.; Yévenes, I.; Neira, M. Efecto del ultrasonido endodóntico sobre clorhexidina al 2% en la formación de paracloroanilina. Estudio in vitro. Rev. Clín. Periodoncia Implantol. Rehabil. Oral 2015, 8, 185–191. [Google Scholar] [CrossRef]
- ICH. Q2B: Validation of analytical procedures: Text and methodology. In ICH Harmonised Tripartite Guideline Stability; ICH: Geneva, Switzerland, 2005; pp. 1–13. [Google Scholar]
- ICH. Q14: Analytical procedure development and revision of Q2(R1) analytical validation. In ICH Harmonised Tripartite Guideline; ICH: Geneva, Switzerland, 2018; pp. 1–3. Available online: http://www.ich.org (accessed on 15 September 2023).
- RFE. Real Farmacopea Española: Guía de Normas de Correcta Fabricación de Medicamentos de Uso Humano y Uso Veterinario; RFE: Madrid, Spain, 2015. [Google Scholar]
- AEMPS. Ficha Técnica Cristalmina 10 mg/mL Solución para Pulverización Cutánea; AEMPS: Madrid, Spain, 2021. [Google Scholar]
- AEMPS. Ficha Técnica Cristalmina 10 mg/mL Solución Cutánea; AEMPS: Madrid, Spain, 2017. [Google Scholar]
- Consejo General de Colegios Farmacéuticos. Procedimiento de Formulación Magistral. In Buenas Prácticas en Farmacia Comunitaria; Consejo General de Colegios Farmacéuticos: Madrid, Spain, 2017. [Google Scholar]
- Qiu, F. Practical considerations. In Accelerated Predictive Stability (APS) Fundamentals and Pharmaceutical Industry Practices; Academic Press: Cambridge, MA, USA, 2018; pp. 76–100. [Google Scholar]
- USP 35: USP 35 general chapter <1150>, Pharmaceutical stability 2023; pp. 763–765.
- Waterman, K.C.; Adami, R.C. Accelerated aging: Prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; Carella, A.J.; Gumkowski, M.J.; Lukulay, P.; MacDonald, B.C.; Roy, M.C.; Shamblin, S.L. Improved protocol and data analysis for accelerated shelf-lifecestimation of solid dosage forms. Pharm. Res. 2007, 24, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C. Understanding and predicting pharmaceutical product shelf-life. In Hand Book of Stability Testing in Pharmaceutical Development; Huynh-Ba, K., Ed.; Springer Science and Business: New York, NY, USA, 2009; pp. 115–135. [Google Scholar]
- Carstensen, J.T. Drug Stability, Principles and Practice; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Clancy, D.; Hodnett, N.; Orr, R.; Owen, M.; Peterson, J. Kinetic model development for accelerated stability studies. AAPS PharmSciTech 2017, 18, 1158–1176. [Google Scholar] [CrossRef] [PubMed]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Khar, R.K. Enhanced stability of ramipril in nanoemulsion containing cremophor-EL: A technical note. AAPS PharmSciTech 2008, 9, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.; Elzayat, E.M.; Alhowyan, A.A.; Alshehri, S.; Shakeel, F. Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide. J. Mol. Liq. 2018, 250, 323–328. [Google Scholar] [CrossRef]
- ICH. Q1B: Stability Testing: Photostability Testing of New Drug Substances and Products; ICH: Geneva, Switzerland, 1996. [Google Scholar]
- ICH. Q3A(R2): Impurities in New Drug Substances; ICH: Geneva, Switzerland, 2006. [Google Scholar]
- ICH. Q3B(R2): Impurities in New Drug Products; ICH: Geneva, Switzerland, 2006. [Google Scholar]

| Temperature (°C) | 0 Order | 1st Order | 2nd Order | Avrami | Diffusion |
|---|---|---|---|---|---|
| Formulation | DCHX | ||||
| 50 | 0.988 | 0.981 | 0.973 | 0.997 | 0.863 |
| 60 | 0.093 | 0.086 | 0.080 | 0.856 | 0.295 |
| 70 | 0.966 | 0.976 | 0.978 | 0.951 | 0.772 |
| 75 | 0.928 | 0.937 | 0.94 | 0.959 | 0.856 |
| 80 | 0.582 | 0.457 | 0.336 | 0.819 | 0.647 |
| Mean R2 | 0.711 | 0.688 | 0.662 | 0.932 | 0.686 |
| Formulation | CR | ||||
| 50 | 0.884 | 0.877 | 0.870 | 0.419 | 0.630 |
| 60 | 0.924 | 0.913 | 0.902 | 0.975 | 0.695 |
| 70 | 0.820 | 0.809 | 0.796 | 0.874 | 0.823 |
| 75 | 0.988 | 0.992 | 0.994 | 0.982 | 0.870 |
| 80 | 0.949 | 0.911 | 0.856 | 0.884 | 0.998 |
| Mean R2 | 0.920 | 0.900 | 0.884 | 0.827 | 0.803 |
| Formulation | Degradation Reaction | Ea (Kcal/mol) | R2 |
|---|---|---|---|
| DCHX | Avrami | 18.52 ± 2.61 | 0.941 |
| CR | Zero-order | 24.13 ± 3.19 | 0.989 |
| Environmental Conditions | Color | Material | Weight Loss (g) | Evaporation Rate (%/day) | Average Evaporation Rate (%/day) | |||
|---|---|---|---|---|---|---|---|---|
| (T0–T1) | (T0–T2) | (T0–T3) | (T0–T4) | |||||
| Fridge (5 ± 3 °C) | Clear | Glass | 0.0033 | 0.0341 | 0.0458 | 0.1091 | 0.0003 | 0.0002 |
| 0.0000 | 0.0028 | 0.0152 | 0.0220 | 0.0001 | ||||
| 0.0000 | 0.0086 | 0.0140 | 0.0409 | 0.0001 | ||||
| Clear | Plastic | 0.4202 | 0.8097 | 0.3032 | 0.8320 | 0.0023 | 0.0026 | |
| 0.5314 | 1.0886 | 0.4340 | 1.1458 | 0.0031 | ||||
| 0.3858 | 0.7894 | 0.3112 | 0.8474 | 0.0023 | ||||
| Amber | Glass | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| 0.0088 | 0.0103 | 0.0000 | 0.0083 | 0.0000 | ||||
| 0.0067 | 0.0085 | 0.0000 | 0.0068 | 0.0000 | ||||
| Amber | Plastic | 0.5024 | 1.3434 | 0.6059 | 1.3992 | 0.0038 | 0.0039 | |
| 0.4990 | 1.2241 | 0.5256 | 1.3109 | 0.0036 | ||||
| 0.4979 | 1.3292 | 0.6085 | 1.5196 | 0.0042 | ||||
| Room Temperature (25 ± 2 °C) | Clear | Glass | 0.0000 | 0.0152 | 0.0695 | 0.1085 | 0.0003 | 0.0002 |
| 0.0000 | 0.0000 | 0.0481 | 0.0000 | 0.0000 | ||||
| 0.0125 | 0.0501 | 0.0534 | 0.1416 | 0.0004 | ||||
| Clear | Plastic | 1.2600 | 5.3058 | 2.8915 | 5.9977 | 0.0164 | 0.0122 | |
| 0.8258 | 3.5210 | 1.9177 | 3.8352 | 0.0105 | ||||
| 0.7460 | 4.6935 | 2.0707 | 3.4915 | 0.0096 | ||||
| Amber | Glass | 0.0001 | 0.0043 | 0.0059 | 0.0091 | 0.0000 | 0.0001 | |
| 0.0040 | 0.0064 | 0.0040 | 0.0109 | 0.0000 | ||||
| 0.0394 | 0.0427 | 0.0047 | 0.0439 | 0.0001 | ||||
| Amber | Plastic | 0.4046 | 3.6493 | 2.3016 | 4.4621 | 0.0122 | 0.0095 | |
| 0.4508 | 3.0884 | 1.8447 | 3.6032 | 0.0099 | ||||
| 0.3963 | 2.0552 | 1.1622 | 2.3305 | 0.0064 | ||||
| Oven (30 ± 0.5 °C) | Clear | Glass | 0.0026 | 0.0393 | 0.0516 | 0.0946 | 0.0003 | 0.0003 |
| 0.0252 | 0.0585 | 0.0490 | 0.1283 | 0.0004 | ||||
| 0.0067 | 0.0317 | 0.0357 | 0.0713 | 0.0002 | ||||
| Clear | Plastic | 0.9252 | 4.2107 | 2.3892 | 4.9298 | 0.0135 | 0.0142 | |
| 1.3762 | 4.7572 | 2.4185 | 5.9392 | 0.0163 | ||||
| 1.3266 | 4.3905 | 2.2126 | 4.6557 | 0.0128 | ||||
| Amber | Glass | 0.0009 | 0.0033 | 0.0034 | 0.0070 | 0.0000 | 0.0000 | |
| 0.0011 | 0.0039 | 0.0041 | 0.0087 | 0.0000 | ||||
| 0.0019 | 0.0059 | 0.0061 | 0.0121 | 0.0000 | ||||
| Amber | Plastic | 0.5389 | 8.0568 | 5.4851 | 10.8609 | 0.0298 | 0.0202 | |
| 0.5515 | 4.0546 | 2.5390 | 5.0225 | 0.0138 | ||||
| 0.5709 | 4.8603 | 3.1353 | 6.2667 | 0.0172 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, O.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life. Molecules 2023, 28, 7925. https://doi.org/10.3390/molecules28237925
González-González O, Ballesteros MP, Torrado JJ, Serrano DR. Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life. Molecules. 2023; 28(23):7925. https://doi.org/10.3390/molecules28237925
Chicago/Turabian StyleGonzález-González, Olga, M. Paloma Ballesteros, Juan J. Torrado, and Dolores R. Serrano. 2023. "Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life" Molecules 28, no. 23: 7925. https://doi.org/10.3390/molecules28237925