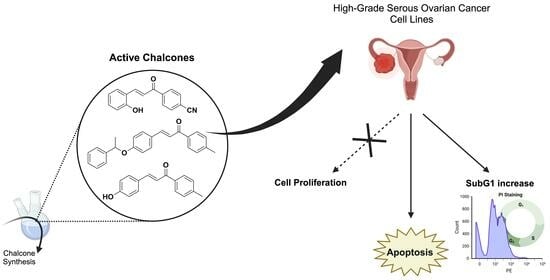
Targeting Ovarian Cancer with Chalcone Derivatives: Cytotoxicity and Apoptosis Induction in HGSOC Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cytotoxicity Levels of Chalcone-Derivative Compounds In Vitro
2.3. Cell Cycle Analysis by PI Staining
2.4. Chalcones 3a, 3h, and 3i Induced Apoptotic Cell Death in OVCAR-3 Cells
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures
3.2.1. General Synthetic Procedure for Title Compounds 3a–3h
3a. (E)-4-(3-(2-hydroxyphenyl)acryloyl)benzonitrile
3b. (E)-4-(3-(4-(dimethylamino)phenyl)acryloyl)benzonitrile
3c. (E)-4-(3-(benzo[d][1,3]dioxol-5-yl)acryloyl)benzonitrile
3d. (E)-3-(4-(pyrrolidin-1-yl)phenyl)-1-(p-tolyl)prop-2-en-1-one [26]
3e. (E)-3-(benzo[d][1,3]dioxol-5-yl)-1-(p-tolyl)prop-2-en-1-one
3f. (E)-4-(3-(4-(benzyloxy)phenyl)acryloyl)benzonitrile
3g. (E)-3-(4-(dimethylamino)phenyl)-1-phenylprop-2-en-1-one [27]
3h. (E)-3-(4-hydroxyphenyl)-1-(p-tolyl)prop-2-en-1-one [28]
3.2.2. Synthesis of (E)-3-(4-(1-phenylethoxy)phenyl)-1-(p-tolyl)prop-2-en-1-one (3i)
3.3. Cell Culture
3.4. NCI-Sulforhodamine B (SRB) Cell Viability Assay
3.5. Cell Cycle Analysis with PI Staining
3.6. Annexin-V/7-AAD Apoptosis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, Q.; Han, L.; Liu, X. Systematic analysis and prediction for disease burden of ovarian cancer attributable to hyperglycemia: A comparative study between China and the world from 1990 to 2019. Front. Med. 2023, 10, 1145487. [Google Scholar] [CrossRef]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Subtypes of Ovarian Cancer and Ovarian Cancer Screening. Diagnostics 2017, 7, 12. [Google Scholar] [CrossRef]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Tsonis, O.; Gkrozou, F.; Vlachos, K.; Paschopoulos, M.; Mitsis, M.C.; Zakynthinakis-Kyriakou, N.; Boussios, S.; Pappas-Gogos, G. Upfront debulking surgery for high-grade serous ovarian carcinoma: Current evidence. Ann. Transl. Med. 2020, 8, 1707. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and Treatment of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A.; et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef]
- Hockings, H.; Miller, R.E. The role of PARP inhibitor combination therapy in ovarian cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231173183. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef]
- Alkema, N.G.; Wisman, G.B.; van der Zee, A.G.; van Vugt, M.A.; de Jong, S. Studying platinum sensitivity and resistance in high-grade serous ovarian cancer: Different models for different questions. Drug Resist. Updat. 2016, 24, 55–69. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2014, 15, 87–120. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Moriello, A.S.; Luongo, L.; Guida, F.; Christodoulou, M.S.; Perdicchia, D.; Passarella, D.; Di Marzo, V.; De Petrocellis, L. Chalcone Derivatives Activate and Desensitize the Transient Receptor Potential Ankyrin 1 Cation Channel, Subfamily A, Member 1 TRPA1 Ion Channel: Structure-Activity Relationships in vitro and Anti-nociceptive and Anti-inflammatory Activity in vivo. CNS Neurol. Disord. Drug Targets 2016, 15, 987–994. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Sahin, I.D.; Christodoulou, M.S.; Guzelcan, E.A.; Koyas, A.; Karaca, C.; Passarella, D.; Cetin-Atalay, R. A small library of chalcones induce liver cancer cell death through Akt phosphorylation inhibition. Sci. Rep. 2020, 10, 11814. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; El-Shorbagy, H.M.; Hassaneen, H.M.; Abdelhamid, I.A.; Sabet, S.; Ibrahim, S.A. [1,2,4] Triazolo [3,4-a]isoquinoline chalcone derivative exhibits anticancer activity via induction of oxidative stress, DNA damage, and apoptosis in Ehrlich solid carcinoma-bearing mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 1225–1238. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef]
- Corsini, E.; Facchetti, G.; Esposito, S.; Maddalon, A.; Rimoldi, I.; Christodoulou, M.S. Antiproliferative effects of chalcones on T cell acute lymphoblastic leukemia-derived cells: Role of PKCβ. Arch. Pharm. 2020, 353, e2000062. [Google Scholar] [CrossRef]
- Quaglio, D.; Zhdanovskaya, N.; Tobajas, G.; Cuartas, V.; Balducci, S.; Christodoulou, M.S.; Fabrizi, G.; Gargantilla, M.; Priego, E.-M.; Carmona Pestaña, A.; et al. Chalcones and Chalcone-mimetic Derivatives as Notch Inhibitors in a Model of T-cell Acute Lymphoblastic Leukemia. ACS Med. Chem. Lett. 2019, 10, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, L.G.; Pinzi, L.; Facchetti, G.; Bortolini, B.; Christodoulou, M.S.; Binda, C.; Rastelli, G.; Rimoldi, I.; Passarella, D.; Di Paolo, M.L.; et al. Promising Non-cytotoxic Monosubstituted Chalcones to Target Monoamine Oxidase-B. ACS Med. Chem. Lett. 2021, 12, 1151–1158. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com (accessed on 15 November 2023).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytom. A 2007, 71, 125–131. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Ibnaouf, K.H. Synthesis of novel pyrrolidinyl chalcone derivatives: Spectral properties, energy band-gap tailoring, and amplified spontaneous emission. J. Mol. Struct. 2022, 1250, 131698. [Google Scholar] [CrossRef]
- Hall, M.J.; McDonnell, S.O.; Killoran, J.; O’Shea, D.F. A modular synthesis of unsymmetrical tetraarylazadipyrromethenes. J. Org. Chem. 2005, 70, 5571–5578. [Google Scholar] [CrossRef]
- Bai, X.; Shi, W.Q.; Chen, H.F.; Zhang, P.; Li, Y.; Yin, S.F. Synthesis and antitumor activity of 1-acetyl-3-(4-phenyl)-4,5-dihydro-2-pyrazoline-5-phenylursolate and 4-chalcone ursolate derivatives. Chem. Nat. Compd. 2012, 48, 60–65. [Google Scholar] [CrossRef]




| Compound | IC50 (µM) a | Physicochemical Properties | ||||
|---|---|---|---|---|---|---|
| OVCAR-3 | OVSAHO | KURAMOCHI | clogP c | n HBD d | n HBA e | |
| 3a | 7.1 ± 1.7 | 16.2 ± 0.9 | 9.7 ± 0.7 | 3.3 | 1 | 3 |
| 3b | NI b | NI b | 39.2 ± 28.8 | 3.7 | 0 | 2 |
| 3c | 33.5 ± 4.1 | NI b | NI b | 3.5 | 0 | 4 |
| 3d | NI b | NI b | 15.7 ± 0.2 | 4.8 | 0 | 1 |
| 3e | 13.8 ± 1.5 | 27.3 ± 3.8 | NI b | 4.2 | 0 | 3 |
| 3f | NI b | NI b | NI b | 5.2 | 0 | 3 |
| 3g | 14.4 ± 1.3 | 38.4 ± 2.8 | 13.8 ± 0.6 | 3.9 | 0 | 1 |
| 3h | 20.8 ± 3.1 | 17.8 ± 1.6 | 15.1 ± 0.7 | 3.8 | 1 | 2 |
| 3i | 21.7 ± 2.4 | 24.3 ± 1.8 | 11.3 ± 1.4 | 6.3 | 0 | 2 |
| Carboplatin | 20.9 ± 2.2 | 27.7 ± 0.9 | 32 ± 1.3 | |||
| Olaparib | 75.8 ± 6.1 | 97.3 ± 1.7 | 84.5 ±11.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merve Aydin, E.; Canıtez, İ.S.; Colombo, E.; Princiotto, S.; Passarella, D.; Dallavalle, S.; Christodoulou, M.S.; Durmaz Şahin, I. Targeting Ovarian Cancer with Chalcone Derivatives: Cytotoxicity and Apoptosis Induction in HGSOC Cells. Molecules 2023, 28, 7777. https://doi.org/10.3390/molecules28237777
Merve Aydin E, Canıtez İS, Colombo E, Princiotto S, Passarella D, Dallavalle S, Christodoulou MS, Durmaz Şahin I. Targeting Ovarian Cancer with Chalcone Derivatives: Cytotoxicity and Apoptosis Induction in HGSOC Cells. Molecules. 2023; 28(23):7777. https://doi.org/10.3390/molecules28237777
Chicago/Turabian StyleMerve Aydin, Elif, İdil Su Canıtez, Eleonora Colombo, Salvatore Princiotto, Daniele Passarella, Sabrina Dallavalle, Michael S. Christodoulou, and Irem Durmaz Şahin. 2023. "Targeting Ovarian Cancer with Chalcone Derivatives: Cytotoxicity and Apoptosis Induction in HGSOC Cells" Molecules 28, no. 23: 7777. https://doi.org/10.3390/molecules28237777