Barium Lanthanum Oxide Nanosheets in Photocatalytic and Forensic Applications: One-Pot Synthesis and Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. PXRD Analysis
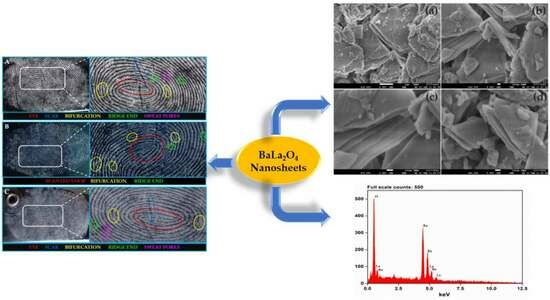
2.2. Morphology
2.3. DRS Studies
2.4. Fluorescence Studies
2.5. Chromaticity Analysis
2.6. Latent Fingerprints (LFPs) Visualization
2.7. MR Degradation by BaLa2O4 NSs
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of BaLa2O4 Nanosheets
3.3. Photocatalytic Degradation Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Majani, S.S.; Gowda, B.G.; Prema, R.; Usha, V.; Shivamallu, C.; Iqbal, M.; Amachawadi, R.G.; Venkatachalaiah, K.; Kollur, S.P. Dysprosium doped BaMgO2 nanopowders as highly luminescent materials: Preparation, characterization, and forensic application. Inorg. Chem. Commun. 2023, 155, 111133. [Google Scholar] [CrossRef]
- Majani, S.S.; Basavaraj, R.; Venkatachalaiah, K.; Kollur, S.P.; Chandrasekhar, T. Versatile deep red-emitting SrCeO3: Eu3+ nanopowders for display devices and advanced forensic applications. J. Solid State Chem. 2023, 329, 124360. [Google Scholar] [CrossRef]
- Yang, K.; Tang, H.; Jiao, Y.; Gao, L.; Zhang, M.; Qin, J.; Li, W.; Lu, S.; He, Y. An AIE-active orange-emitting cationic iridium(III) complex for latent fingerprints detection via a simple powder dusting method. J. Lumin. 2023, 257, 119721. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Nanomaterials for latent fingerprint detection: A review. J. Mater. Res. Technol. 2021, 12, 1856–1885. [Google Scholar] [CrossRef]
- Singla, N.; Kaur, M.; Sofat, S. Automated latent fingerprint identification system: A review. Forensic Sci. Int. 2020, 309, 110187. [Google Scholar] [CrossRef]
- Pushpendra; Suryawanshi, I.; Kalia, R.; Kunchala, R.K.; Mudavath, S.L.; Naidu, B.S. Detection of latent fingerprints using luminescent Gd0.95Eu0.05PO4 nanorods. J. Rare Earths 2021, 40, 572–578. [Google Scholar] [CrossRef]
- Trabelsi, H.; Akl, M.; Akl, S.H. Ultrasound assisted Eu3+–doped strontium titanate nanophosphors: Labeling agent useful for visualization of latent fingerprints. Powder Technol. 2021, 384, 70–81. [Google Scholar] [CrossRef]
- Yuan, C.; Li, M.; Wang, M.; Zhang, L. Cationic dye-diatomite composites: Novel dusting powders for developing latent fingerprints. Dye. Pigment. 2018, 153, 18–25. [Google Scholar] [CrossRef]
- Ashwini, K.; Premkumar, H.; Darshan, G.; Prasad, B.D.; Nagabhushana, H.; Sharma, S.; Prashantha, S. Dysprosium doped strontium aluminate dusting powder: Sweat pores visualization and white LED component. Inorg. Chem. Commun. 2021, 134, 109028. [Google Scholar] [CrossRef]
- Niu, X.; Song, T.; Xiong, H. Large scale synthesis of red emissive carbon dots powder by solid-state reaction for fingerprint identification. Chin. Chem. Lett. 2021, 32, 1953–1956. [Google Scholar] [CrossRef]
- Errington, B.; Lawson, G.; Lewis, S.W.; Smith, G.D. Micronised Egyptian blue pigment: A novel near-infrared luminescent fingerprint dusting powder. Dye. Pigment. 2016, 132, 310–315. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, M.; Li, M. Design and synthesis of silica- and silicate-based materials, and their application in the development and analysis of latent fingerprints. TrAC Trends Anal. Chem. 2023, 167, 117278. [Google Scholar] [CrossRef]
- Gomes, F.M.; de Pereira, C.M.P.; Mariotti, K.d.C.; Pereira, T.M.; dos Santos, N.A.; Romão, W. Study of latent fingerprints—A review. Forensic Chem. 2023, 35, 100525. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Lv, X.-S.; Li, H.-D. A luminescent Eu-based MOFs material for the sensitive detection of nitro explosives and development of fingerprint. Inorg. Chem. Commun. 2023, 156, 111267. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Bin Khamidun, M.H. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; Elhusseiny, A.F.; Hussein, S.M.; Sadik, W.A. Sustainable and energy-efficient photocatalytic degradation of textile dye assisted by ecofriendly synthesized silver nanoparticles. Sci. Rep. 2023, 13, 2302. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Singh, L.; Ram, C. Photocatalytic degradation of hazardous Rhodamine B dye using sol-gel mediated ultrasonic hydrothermal synthesized of ZnO nanoparticles. Results Eng. 2023, 17, 100890. [Google Scholar] [CrossRef]
- Renuka, L.; Anantharaju, K.; Gurushantha, K.; Nagabhushana, H.; Vidya, Y.; Suresh, C.; Sennappan, M. Phase-transformation synthesis of Li codoped ZrO2: Eu3+ nanomaterials: Characterization, photocatalytic, luminescent behavior and latent fingerprint development. Ceram. Int. 2021, 47 Pt B, 10332–10345. [Google Scholar] [CrossRef]
- Darshan, G.; Prasad, B.D.; Premkumar, H.; Sharma, S.; Kiran, K.; Nagabhushana, H. 20—Fluorescent quantum dots as labeling agents for the effective detection of latent fingerprints on various surfaces. In Quantum Dots; Woodhead Publishing Series in Electronic and Optical Materials; Kalyani, N.T., Dhoble, S.J., Domanska, M.M., Vengadaesvaran, B., Nagabhushana, H., Arof, A.K., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 539–574. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, H.; Ma, R.; Li, X.; Du, X.; Zhang, M. Use of conductive Ti2O3 nanoparticles for optical and electrochemical imaging of latent fingerprints on various substrates. J. Electroanal. Chem. 2023, 936, 117387. [Google Scholar] [CrossRef]
- Bandi, R.; Kannikanti, H.G.; Dadigala, R.; Gangapuram, B.R.; Vaidya, J.R.; Guttena, V. One step synthesis of hydrophobic carbon dots powder with solid state emission and application in rapid visualization of latent fingerprints. Opt. Mater. 2020, 109, 110349. [Google Scholar] [CrossRef]
- Girisha, H.; Krushna, B.; Prasad, B.P.; Sharma, S.; Srikanth, C.; Kumar, J.B.P.; Nagabhushana, H. A novel single phase La2CaZnO5:Dy3+ phosphor for potential applications in WLED’s, latent fingerprint and cheiloscopy. J. Lumin. 2023, 255, 119539. [Google Scholar] [CrossRef]
- Pavitra, E.; Raju, G.S.R.; Park, J.Y.; Hussain, S.K.; Chodankar, N.R.; Rao, G.M.; Han, Y.-K.; Huh, Y.S. An efficient far-red emitting Ba2LaNbO6:Mn4+ nanophosphor for forensic latent fingerprint detection and horticulture lighting applications. Ceram. Int. 2020, 46, 9802–9809. [Google Scholar] [CrossRef]
- Park, J.Y.; Jang, K.W.; Yang, H.K. Development of red-emitting Ba2LaSbO6:Mn4+ phosphors for latent fingerprint detection. Ceram. Int. 2021, 47, 19496–19504. [Google Scholar] [CrossRef]
- Sehrawat, P.; Khatkar, A.; Boora, P.; Kumar, M.; Singh, S.; Malik, R.K.; Khatkar, S.P.; Taxak, V.B. Fabrication of single-phase BaLaAlO4:Dy3+ nanophosphors by combustion synthesis. Mater. Manuf. Process. 2020, 35, 1259–1267. [Google Scholar] [CrossRef]
- Ikram, M.; Abid, N.; Haider, A.; Ul-Hamid, A.; Haider, J.; Shahzadi, A.; Nabgan, W.; Goumri-Said, S.; Butt, A.R.; Benali Kanoun, M. Toward efficient dye degradation and the bactericidal behavior of Mo-doped La2O3 nanostructures. Nanoscale Adv. 2022, 4, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, N.; Igarashi, K.; Saitoh, H.; Okazaki, S. Preparation of a Voluminous Composite Oxide of BaLa2O4 and Its Catalytic Performance for the Oxidative Coupling of Methane. Bull. Chem. Soc. Jpn. 1993, 66, 1799–1806. [Google Scholar] [CrossRef]
- The Materials Project. Materials Data on BaLa2O4 by Materials Project; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2020.
- Basavaraj, R.B.; Navami, D.; Deepthi, N.; Venkataravanappa, M.; Lokesh, R.; Kumar, K.S.; Sreelakshmi, T.K. Novel orange-red emitting Pr3+ doped CeO2 nanopowders for white light emitting diode applications. Inorg. Chem. Commun. 2020, 120, 108164. [Google Scholar] [CrossRef]
- Sushma, K.C.; Basavaraj, R.; Aarti, D.; Reddy, M.M.; Nagaraju, G.; Rudresha, M.; Kumar, H.S.; Venkatachalaiah, K. Efficient red-emitting SrZrO3:Eu3+ phosphor superstructures for display device applications. J. Mol. Struct. 2023, 1283, 135192. [Google Scholar] [CrossRef]
- Sonika; Han, S.-D.; Khatkar, S.; Kumar, M.; Taxak, V. Sol–gel synthesis, characterization and luminescent properties of Tb3+ doped MLa2O4 (M=Sr or Ba) nanophosphors. Mater. Sci. Eng. B 2013, 178, 1436–1442. [Google Scholar] [CrossRef]
- Marí, B.; Singh, K.; Sahal, M.; Khatkar, S.; Taxak, V.; Kumar, M. Characterization and photoluminescence properties of some MLn2(1−x)O4: 2xEu3+ or 2xTb3+ systems (M = Ba or Sr, Ln = Gd or La). J. Lumin. 2011, 131, 587–591. [Google Scholar] [CrossRef]
- Basavaraj, R.B.; Kumar, S.; Aarti, D.; Nagaraju, G.; Kumar, H.S.; Soundar, R.; Shashidhara, T.; Sumedha, H.; Shahsank, M. Color tunable orange-red light emitting Sm3+ doped BaZrO3 nanopowders: Photoluminescence properties for w-LED applications. Inorg. Chem. Commun. 2021, 128, 108577. [Google Scholar] [CrossRef]
- Navami, D.; Basavaraj, R.; Darshan, G.; Inamdar, H.K.; Sharma, S.; Premkumar, H.; Nagabhushana, H. Evolution of shapes and identification of level II and III features of fingerprints using CaZrO3:Sm3+ fluorescent markers prepared via solution combustion route. Opt. Mater. 2019, 88, 479–487. [Google Scholar] [CrossRef]
- Dewangan, P.; Bisen, D.; Brahme, N.; Sharma, S. Structural characterization and luminescence properties of Dy3+ doped Ca3MgSi2O8 phosphors. J. Alloys Compd. 2019, 777, 423–433. [Google Scholar] [CrossRef]
- Kumar, K.N.; Vijayalakshmi, L.; Hwang, P.; Wadhwani, A.D.; Choi, J. Bright red-luminescence of Eu3+ion-activated La10W22O81 microphosphors for noncytotoxic latent fingerprint imaging. J. Alloys Compd. 2020, 840, 155589. [Google Scholar] [CrossRef]
- Babu, K.V.; Renuka, C.; Basavaraj, R.; Darshan, G.; Nagabhushana, H. One pot synthesis of TiO2:Eu3+ hierarchical structures as a highly specific luminescent sensing probe for the visualization of latent fingerprints. J. Rare Earths 2019, 37, 134–144. [Google Scholar] [CrossRef]
- Sodhi, G.; Kaur, J. Powder method for detecting latent fingerprints: A review. Forensic Sci. Int. 2001, 120, 172–176. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Yao, H.; Ye, Y.; Zhou, J. Development of latent fingerprints by degradable highly-adhering powder—A long-term strategy for the fading of fingerprint residues. Dye. Pigment. 2023, 219, 111597. [Google Scholar] [CrossRef]
- Di, L.; Xing, Y.; Yang, Z.; Li, C.; Yu, Z.; Wang, X.; Xia, Z. High-definition and robust visualization of latent fingerprints utilizing ultrabright aggregation-induced emission of iridium developer. Talanta 2023, 264, 124775. [Google Scholar] [CrossRef]






 represents the point of maximum emission.
represents the point of maximum emission.
 represents the point of maximum emission.
represents the point of maximum emission.



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majani, S.S.; Meghana; S H, S.; J, S.; Umesh, S.; Shivamallu, C.; Iqbal, M.; Amachawadi, R.G.; K N, V.; Kollur, S.P. Barium Lanthanum Oxide Nanosheets in Photocatalytic and Forensic Applications: One-Pot Synthesis and Characterization. Molecules 2023, 28, 7228. https://doi.org/10.3390/molecules28207228
Majani SS, Meghana, S H S, J S, Umesh S, Shivamallu C, Iqbal M, Amachawadi RG, K N V, Kollur SP. Barium Lanthanum Oxide Nanosheets in Photocatalytic and Forensic Applications: One-Pot Synthesis and Characterization. Molecules. 2023; 28(20):7228. https://doi.org/10.3390/molecules28207228
Chicago/Turabian StyleMajani, Sanjay S., Meghana, Sowmyashree S H, Sowjanyashree J, Sahaja Umesh, Chandan Shivamallu, Muzaffar Iqbal, Raghavendra G. Amachawadi, Venkatachalaiah K N, and Shiva Prasad Kollur. 2023. "Barium Lanthanum Oxide Nanosheets in Photocatalytic and Forensic Applications: One-Pot Synthesis and Characterization" Molecules 28, no. 20: 7228. https://doi.org/10.3390/molecules28207228