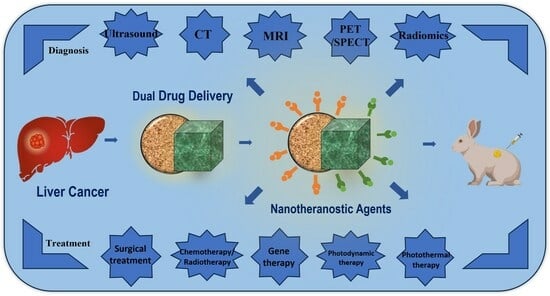
Review of the Application of Dual Drug Delivery Nanotheranostic Agents in the Diagnosis and Treatment of Liver Cancer
Abstract
:1. Introduction
2. Diagnosis and Treatment of Liver Cancer
2.1. Imaging Diagnosis of Liver Cancer
2.2. Treatment of Liver Cancer
3. Nano-Diagnostic Technology and Its Application for Tumor Treatment
3.1. Nano-Diagnostic and Therapeutic Technology
3.2. Nano-Diagnostic Agent
4. Research Progress on Dual-Drug-Delivery Nano-Theranostic Agents for Liver Cancer
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.G.; Zhu, J.; Zhang, Y.H.; Chen, Y.S.; Ding, L.L.; Chen, H.Z.; Shen, A.G.; Wang, G.R. Liver Cancer Survival: A Real World Observation of 45 Years with 32,556 Cases. J. Hepatocell. Carcinoma 2021, 8, 1023–1034. [Google Scholar] [CrossRef]
- Li, J.Y.; Yao, M.; Shao, Y.X.; Yao, D.F. The application of bio-nanotechnology in tumor diagnosis and treatment: A view. Nanotechnol. Rev. 2018, 7, 257–266. [Google Scholar] [CrossRef]
- Kher, C.; Kumar, S. The Application of Nanotechnology and Nanomaterials in Cancer Diagnosis and Treatment: A Review. Cureus J. Med. Sci. 2022, 14, e29059. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kohli, M.; Smith, A. Nanoparticles for Combination Drug Therapy. Acs Nano 2013, 7, 9518–9525. [Google Scholar] [CrossRef]
- Li, Y.; Thambi, T.; Lee, D.S. Co-Delivery of Drugs and Genes Using Polymeric Nanoparticles for Synergistic Cancer Therapeutic Effects. Adv. Healthc. Mater. 2018, 7, 1700886. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Chen, Y.; Li, Y.H.; Yin, X.B. Magnetic Resonance Imaging-Guided Multi-Drug Chemotherapy and Photothermal Synergistic Therapy with pH and NIR-Stimulation Release. Acs Appl. Mater. Interfaces 2017, 9, 22278–22288. [Google Scholar] [CrossRef]
- Liao, L.Y.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A Convergent Synthetic Platform for Single-Nanoparticle Combination Cancer Therapy: Ratiometric Loading and Controlled Release of Cisplatin, Doxorubicin, and Camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Quadir, M.A.; Haag, R. Biofunctional nanosystems based on dendritic polymers. J. Control. Release 2012, 161, 484–495. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym.-Plast. Technol. Mater. 2021, 60, 1996–2024. [Google Scholar] [CrossRef]
- Chang, H.; Kim, J.; Rho, W.Y.; Pham, X.H.; Lee, J.H.; Lee, S.H.; Jeong, D.H.; Jun, B.H. Silica Nanoparticles. In Nanotechnology for Bioapplications; Jun, B.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1309, pp. 41–65. [Google Scholar]
- Luo, J.; Yang, J.; Li, Y.; He, L.; Jiang, B. Synthesis of Amphiphilic Silica Nanoparticles with Double-sphere Morphology. Chem. J. Chin. Univ.-Chin. 2018, 39, 2170–2177. [Google Scholar] [CrossRef]
- Li, Y.A.; Li, W.; Bao, W.; Liu, B.; Li, D.; Jiang, Y.M.; Wei, W.; Ren, F.Z. Bioinspired peptosomes with programmed stimuli-responses for sequential drug release and high-performance anticancer therapy. Nanoscale 2017, 9, 9317–9324. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Niu, M.; Chen, X.W.; Tan, L.F.; Fu, C.H.; Ren, X.L.; Ren, J.; Li, L.F.; Xu, K.; Zhong, H.S.; et al. Biocompatible and biodegradable zeolitic imidazolate framework/polydopamine nanocarriers for dual stimulus triggered tumor thermo-chemotherapy. Biomaterials 2018, 162, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yan, D.; Xiao, Q.; Zhang, G. Correlations of ultrasonic features with severity of liver cancer and p16 expression in patients with liver cancer. Neoplasma 2019, 66, 149–154. [Google Scholar] [CrossRef]
- Durot, I.; Wilson, S.R.; Willmann, J.K. Contrast-enhanced ultrasound of malignant liver lesions. Abdom. Radiol. 2018, 43, 819–847. [Google Scholar] [CrossRef]
- Almotairi, S.; Kareem, G.; Aouf, M.; Almutairi, B.; Salem, M.A.M. Liver Tumor Segmentation in CT Scans Using Modified SegNet. Sensors 2020, 20, 1516. [Google Scholar] [CrossRef]
- Popovic, P.; Leban, A.; Kregar, K.; Garbajs, M.; Dezman, R.; Bunc, M. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial chemoembolization of hepatocellular carcinoma. Radiol. Oncol. 2018, 52, 14–22. [Google Scholar] [CrossRef]
- Jing, M.Y.; Sun, J.C.; Xi, H.Z.; Liu, Z.H.; Zhang, S.; Deng, L.N.; Han, T.; Zhang, B.; Lin, X.Q.; Zhou, J.L. Abdominal virtual non-contrast images derived from energy spectrum CT to evaluate chemotherapy-related fatty liver disease. Quant. Imaging Med. Surg. 2022, 13, 669–681. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Parakh, A.; Patino, M.; Kambadakone, A.; Rummeny, E.J.; Sahani, D.V. Iodine material density images in dual-energy CT: Quantification of contrast uptake and washout in HCC. Abdom. Radiol. 2018, 43, 3317–3323. [Google Scholar] [CrossRef]
- Morgan, D.E. The Role of Dual-Energy Computed Tomography in Assessment of Abdominal Oncology and Beyond. Radiol. Clin. North Am. 2018, 56, 565–585. [Google Scholar] [CrossRef]
- Grant, M.J.; Didier, R.A.; Stevens, J.S.; Beyder, D.D.; Hunter, J.G.; Thomas, C.R.; Coakley, F.V. Radiation-induced liver disease as a mimic of liver metastases at serial PET/CT during neoadjuvant chemoradiation of distal esophageal cancer. Abdom. Imaging 2014, 39, 963–968. [Google Scholar] [CrossRef]
- Yao, S.-Z.; Zhang, C.-Q.; Chen, J.; Liu, Q.-W.; Li, Q.-G. Clinical evaluation of 18F-FDE PET-CT in detecting malignant liver tumors. Di 1 Jun Yi Da Xue Xue Bao = Acad. J. First Med. Coll. PLA 2003, 23, 1214–1216. [Google Scholar]
- Hyun, S.H.; Eo, J.S.; Lee, J.W.; Choi, J.Y.; Lee, K.H.; Na, S.J.; Hong, I.K.; Oh, J.K.; Chung, Y.A.; Song, B.I.; et al. Prognostic value of F-18-fluorodeoxyglucose positron emission tomography/computed tomography in patients with Barcelona Clinic Liver Cancer stages 0 and A hepatocellular carcinomas: A multicenter retrospective cohort study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Elboga, U.; Celen, Y.Z.; Sever, O.N.; Cayirli, Y.B.; Cimen, U. Comparison of Ga-68-DOTA-FAPI and (18)FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur. J. Radiol. 2021, 142, 109867. [Google Scholar] [CrossRef]
- Zeng, M.S.; Ye, H.Y.; Guo, L.; Peng, W.J.; Lu, J.P.; Teng, G.J.; Huan, Y.; Li, P.; Xu, J.R.; Liang, C.H.; et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging for focal liver lesions in Chinese patients: A multicenter, open-label, phase III study. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Salvaggio, G.; Furlan, A.; Agnello, F.; Cabibbo, G.; Marin, D.; Giannitrapani, L.; Genco, C.; Midiri, M.; Lagalla, R.; Brancatelli, G. Hepatocellular carcinoma enhancement on contrast-enhanced CT and MR imaging: Response assessment after treatment with sorafenib: Preliminary results. Radiol. Medica 2014, 119, 215–221. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Shen, Y.C.; Yu, C.W.; Hsu, C.U.; Hu, F.C.; Hsu, C.H.; Chen, B.B.; Wei, S.Y.; Cheng, A.L.; Shih, T.T.F. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J. Hepatol. 2011, 55, 858–865. [Google Scholar] [CrossRef]
- Davenport, M.S.; Khalatbari, S.; Liu, P.S.C.; Maturen, K.E.; Kaza, R.K.; Wasnik, A.P.; Al-Hawary, M.M.; Glazer, D.I.; Stein, E.B.; Patel, J.; et al. Repeatability of Diagnostic Features and Scoring Systems for Hepatocellular Carcinoma by Using MR Imaging. Radiology 2014, 272, 132–142. [Google Scholar] [CrossRef]
- Starmans, M.P.A.; Miclea, R.L.; van der Voort, S.R.; Niessen, W.J.; Thomeer, M.G.; Klein, S. Classification of malignant and benign liver tumors using a radiomics approach. In Proceedings of the Conference on Medical Imaging—Image Processing, Houston, TX, USA, 11–13 February 2018. [Google Scholar]
- Jeong, W.K.; Jamshidi, N.; Felker, E.R.; Raman, S.S.; Lu, D.S. Radiomics and radiogenomics of primary liver cancers. Clin. Mol. Hepatol. 2019, 25, 21–29. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, D.S.; Kim, H.J.; Sirlin, C.B.; Chan, M.G.; Korn, R.L.; Rutman, A.M.; Siripongsakun, S.; Lu, D.; Imanbayev, G.; et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 2015, 62, 792–800. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.M.; Zhang, L.; Zhao, Y.M.; Zhang, N.; Wang, L.R.; Zhu, W.P.; He, X.G.; Zhu, H.X.; Xu, W.Q.; et al. Surgical treatment of ovarian cancer liver metastasis. Hepatobiliary Surg. Nutr. 2019, 8, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Hasegawa, K. Advances in the surgical treatment of liver cancer. Biosci. Trends 2022, 16, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Tsung, A. ASO Author Reflections: The Evolution of Minimally Invasive Liver Surgery and the Future with Robotics. Ann. Surg. Oncol. 2018, 25, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Lu, S.C. Chemotherapy-associated liver injury in colorectal cancer. Ther. Adv. Gastroenterol. 2020, 13, 1756284820924194. [Google Scholar] [CrossRef]
- Kohler, B.C.; Waldburger, N.; Schlamp, K.; Jager, D.; Weiss, K.H.; Schulze-Bergkamen, H.; Schirmacher, P.; Springfeld, C. Liver cancers with stem/progenitor-cell features—A rare chemotherapy-sensitive malignancy. Oncotarget 2017, 8, 59991–59998. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Liu, S.S.; Gao, F.; Zou, Y.W.; Ren, Z.G.; Yu, Z.J. The role of tumor microenvironment reprogramming in primary liver cancer chemotherapy resistance. Front. Oncol. 2022, 12, 1008902. [Google Scholar] [CrossRef]
- Ma, J.Y.; Wang, B.Z.; Shao, H.B.; Zhang, S.G.; Chen, X.Z.; Li, F.Z.; Liang, W.Q. Hydrogels for localized chemotherapy of liver cancer: A possible strategy for improved and safe liver cancer treatment. Drug Deliv. 2022, 29, 1457–1476. [Google Scholar] [CrossRef]
- Chen, W.Q.; Chiang, C.L.; Dawson, L.A. Efficacy and safety of radiotherapy for primary liver cancer. Chin. Clin. Oncol. 2021, 10. [Google Scholar] [CrossRef]
- Miura, H.; Ozawa, S.; Nakao, M.; Doi, Y.; Kubo, K.; Kenjo, M.; Nagata, Y. Impact on liver position under breath-hold by computed tomography contrast agents in stereotactic body radiotherapy of liver cancer. Rep. Pract. Oncol. Radiother. 2021, 26, 1035–1044. [Google Scholar] [CrossRef]
- Fukumitsu, N.; Okumura, T.; Sakurai, H. Radiotherapy for liver cancer. J. Gen. Fam. Med. 2017, 18, 126–130. [Google Scholar] [CrossRef]
- Sangro, B.; Herraiz, M.; Prieto, J. Gene therapy of neoplastic liver diseases. Int. J. Biochem. Cell Biol. 2003, 35, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Panis, Y.; Charre, L.; Soubrane, O. Gene therapy for Liver tumors. J. De Chir. 2000, 137, 317–324. [Google Scholar] [CrossRef]
- Zhu, J.X.; Wang, Y.F.; Yang, P.P.; Liu, Q.H.; Hu, J.H.; Yang, W.; Liu, P.; He, F.; Bai, Y.X.; Gai, S.L.; et al. GPC3-targeted and curcumin-loaded phospholipid microbubbles for sono-photodynamic therapy in liver cancer cells. Colloids Surf. B-Biointerfaces 2021, 197, 111358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Zhu, W.T.; Hu, J.H.; Yang, W.; Liu, P.; Liu, Q.H.; Bai, Y.X.; Xie, R. Curcumin-loaded poly(l-lactide-co-glycolide) microbubble-mediated sono-photodynamic therapy in liver cancer cells. Ultrasound Med. Biol. 2020, 46, 2030–2043. [Google Scholar] [CrossRef]
- Hu, J.H.; Shi, J.L.; Gao, Y.Q.; Yang, W.; Liu, P.; Liu, Q.H.; He, F.; Wang, C.X.; Li, T.; Xie, R.; et al. 808 nm Near-Infrared Light-Excited UCNPs@mSio(2)-Ce6-GPC3 Nanocomposites For Photodynamic Therapy In Liver Cancer. Int. J. Nanomed. 2019, 14, 10009–10021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chang, Z.M.; Shao, D.; Zhang, F.; Chen, F.M.; Li, L.; Ge, M.F.; Hu, R.; Zheng, X.; Wang, Y.S.; et al. Janus Gold Triangle-Mesoporous Silica Nanoplatforms for Hypoxia-Activated Radio-Chemo-Photothermal Therapy of Liver Cancer. Acs Appl. Mater. Interfaces 2019, 11, 34755–34765. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, J.Q.; Tian, J.H.; Li, H.G. A polydopamine nanomedicine used in photothermal therapy for liver cancer knocks down the anti-cancer target NEDD8-E3 ligase ROC1 (RBX1). J. Nanobiotechnol. 2021, 19, 323. [Google Scholar] [CrossRef]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef]
- Ahmed, S.; Strand, S.; Weinmann-Menke, J.; Urbansky, L.; Galle, P.R.; Neumann, H. Molecular endoscopic imaging in cancer. Dig. Endosc. 2018, 30, 719–729. [Google Scholar] [CrossRef]
- Kateb, B.; Chiu, K.; Black, K.L.; Yamamoto, V.; Khalsa, B.; Ljubimova, J.Y.; Ding, H.; Patil, R.; Portilla-Arias, J.A.; Modo, M.; et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: What should be the policy? Neuroimage 2011, 54, S106–S124. [Google Scholar] [CrossRef]
- Waaijer, S.J.H.; Kok, I.C.; Eisses, B.; Schroder, C.P.; Jalving, M.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Vries, E.G.E. Molecular Imaging in Cancer Drug Development. J. Nucl. Med. 2018, 59, 726–732. [Google Scholar] [CrossRef]
- Laer, S. Pharmacokinetic and pharmacodynamic modelling in paediatric drug development with a focus on physiology-based pharmacokinetic simulations. Z. Fur Evidenz Fortbild. Und Qual. Im Gesundheitswesen 2019, 141, 66–73. [Google Scholar] [CrossRef]
- Dong, H.; Gao, Y.; Sinko, P.J.; Wu, Z.; Xu, J.; Jia, L. The nanotechnology race between China and the United States. Nano Today 2016, 11, 7–12. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Peppas, N.A. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Deliv. Rev. 2009, 61, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Piccirillo, C.; Mandriota, G.; Di Corato, R. Nanoheterostructures (NHS) and Their Applications in Nanomedicine: Focusing on In Vivo Studies. Materials 2019, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Naleway, M.A.; Pang, A.J.; Chon, K.J.; Taratula, O. A Multifunctional Theranostic Platform Based on Phthalocyanine-Loaded Dendrimer for Image-Guided Drug Delivery and Photodynamic Therapy. Mol. Pharm. 2013, 10, 3946–3958. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef]
- Li, X.H.; He, S.K.; Ma, B.Y. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Liu, J.J.; Chen, Q.; Feng, L.Z.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Kim, N.R.; Chae, C.B. Novel Modulation Techniques using Isomers as Messenger Molecules for Nano Communication Networks via Diffusion. Ieee J. Sel. Areas Commun. 2013, 31, 847–856. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.U.; Husen, A. Biogenic Fabrication of Iron/Iron Oxide Nanoparticles and Their Application. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Heltina, D.; Wulan, P.; Slamet, S. Photocatalytic Activity of Titania Nanotube (Tint)-Carbon Nanotube (CNT) Composite for Degradation of Phenol. In Proceedings of the 3rd International Seminar on Fundamental and Application of Chemical Engineering (ISFAChE), East Java, Indonesia, 1–2 November 2016. [Google Scholar]
- Tarfaoui, M.; Lafdi, K.; El Moumen, A. Mechanical properties of carbon nanotubes based polymer composites. Compos. Part B-Eng. 2016, 103, 113–121. [Google Scholar] [CrossRef]
- Tinwala, H.; Wairkar, S. Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 97, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Simpson, A.L.; Do, R.K.G.; Gonen, M.; Shia, J.; Allen, P.J.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Jarnagin, W.R. Cholangiocarcinoma: Correlation between Molecular Profiling and Imaging Phenotypes. PLoS ONE 2015, 10, e0132953. [Google Scholar] [CrossRef]
- Medavenkata, S.P.; Akshatha, H.S. Nano theranostics—A breakthrough in cancer diagnosis and treatment and regulations of nano technology products. Int. J. Pharm. Sci. Res. 2018, 9, 3136–3149. [Google Scholar] [CrossRef]
- Huang, L.; Chaurasiya, B.; Wu, D.; Wang, H.; Du, Y.; Tu, J.; Webster, T.J.; Sun, C. Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomed.-Nanotechnol. Biol. Med. 2018, 14, 1005–1017. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, L.; Han, Q.; Chen, X.; Li, S.; Li, L.; Su, Z.; Wang, C. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials 2018, 181, 113–125. [Google Scholar] [CrossRef]
- Yao, W.; Liu, C.; Wang, N.; Zhou, H.; Chen, H.; Qiao, W. An MRI-guided targeting dual-responsive drug delivery system for liver cancer therapy. J. Colloid Interface Sci. 2021, 603, 783–798. [Google Scholar] [CrossRef]
- Yan, J.; Xie, S.; Xia, Q.; Li, X.; Chen, S.; Shen, J. Engineering of combination drug delivery of pH/reduction response potential nanocarrier for the treatment of liver cancer. Appl. Nanosci. 2022, 12, 1545–1556. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Xu, X.; Chen, Z.; Ma, L.; Wang, Y.; Guo, X.; Li, J.; Wang, X. Construction of pH-sensitive targeted micelle system co-delivery with curcumin and dasatinib and evaluation of anti-liver cancer. Drug Deliv. 2022, 29, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Lu, Z.; Chang, C.; Zhang, Y.; Li, Y.; Yi, M.; Xiong, B.; Lu, B. Lactobionic acid-functionalized hollow mesoporous silica nanoparticles for cancer chemotherapy and phototherapy. Process Biochem. 2022, 121, 698–706. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Xu, W.; Li, Z.; Wang, L. Synergic fabrication of multifunctional liposomes nanocomposites for improved radiofrequency ablation combination for liver metastasis cancer therapy. Drug Deliv. 2022, 29, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Thi Tuong Vy, P.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.-C. Thermo-Sensitive Poly (N-isopropylacrylamide-co-polyacrylamide) Hydrogel for pH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; Shen, Z.-C.; Jiang, J.-L.; Zhang, B.-C.; Zhang, W.-Z.; Zou, J.-J.; Lin, J.-F.; Li, C.; Shao, J.-W. A multifunctional theranostics nanosystem featuring self-assembly of alcohol-abuse drug and photosensitizers for synergistic cancer therapy. Biomater. Sci. 2022, 10, 6267–6281. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, X.; Zhang, D.; Cui, J.; Zhang, X.; Duan, X.; Trant, J.F.; Li, Y. A nanodiamond chemotherapeutic folate receptor-targeting prodrug with triggerable drug release. Int. J. Pharm. 2023, 630, 122432. [Google Scholar] [CrossRef]
- Thirupathi, K.; Phan, T.T.V.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.-C. pH and Thermoresponsive PNIPAm-co-Polyacrylamide Hydrogel for Dual Stimuli-Responsive Controlled Drug Delivery. Polymers 2023, 15, 167. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H.; Salehi, R. Dual anticancer drug delivery of D-galactose-functionalized stimuli-responsive nanogels for targeted therapy of the liver hepatocellular carcinoma. Eur. Polym. J. 2022, 167, 111061. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Guo, M.; Xu, W.; Wang, J.; Wang, Y.; Suo, A. Hydrazided hyaluronan/cisplatin/indocyanine green coordination nanoprodrug for photodynamic chemotherapy in liver cancer. Carbohydr. Polym. 2022, 276, 118810. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, W.; Chen, W.; Chen, Y.; Huang, Q.; Bao, Q.; Lin, T.; Wang, L.; Zhang, S. Construction and Biological Evaluation of Multiple Modification Hollow Mesoporous Silicone Doxorubicin Nanodrug Delivery System. AAPS PharmSciTech 2022, 23, 180. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Zhang, C.; Zhang, Z.-J.; Xia, L.; Rao, K.-Q.; Gu, L.-H.; Wu, Y.-C.; Lv, Z.-C.; Wu, H.-X.; Zuo, X.-L.; et al. Aggregation-Induced Emission (AIE) and Magnetic Resonance Imaging Characteristics for Targeted and Image-Guided siRNA Therapy of Hepatocellular Carcinoma. Adv. Healthc. Mater. 2022, 11, 2200579. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cui, W.; Prasad, C.V.N.S.V.; Wang, B. Design and Synthesis of Lactose, Galactose and Cholic Acid Related Dual Conjugated Chitosan Derivatives as Potential Anti Liver Cancer Drug Carriers. Polymers 2021, 13, 2939. [Google Scholar] [CrossRef] [PubMed]
- Amoyav, B.; Bloom, A.I.; Goldstein, Y.; Miller, R.; Sharam, M.; Fluksman, A.; Benny, O. Drug-Eluting Porous Embolic Microspheres for Trans-Arterial Delivery of Dual Synergistic Anticancer Therapy for the Treatment of Liver Cancer. Adv. Healthc. Mater. 2023, 2301548. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhang, L.; Zhao, G.; Xu, S.; Li, L.; Su, Z.; Liu, R.; Wang, C. An EPR-independent therapeutic strategy: Cancer cell-mediated dual-drug delivery depot for diagnostics and prevention of hepatocellular carcinoma metastasis. Biomaterials 2021, 268, 120541. [Google Scholar] [CrossRef]
- Qi, C.; Wang, D.; Gong, X.; Zhou, Q.; Yue, X.; Li, C.; Li, Z.; Tian, G.; Zhang, B.; Wang, Q.; et al. Co-Delivery of Curcumin and Capsaicin by Dual-Targeting Liposomes for Inhibition of aHSC-Induced Drug Resistance and Metastasis. Acs Appl. Mater. Interfaces 2021, 13, 16019–16035. [Google Scholar] [CrossRef]
- Arafa, K.K.; Fytory, M.; Mousa, S.A.; El-Sherbiny, I.M. Nanosized biligated metal-organic framework systems for enhanced cellular and mitochondrial sequential targeting of hepatic carcinoma. Biomater. Sci. 2021, 9, 6609–6622. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskaran, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Dual-Functional Iron Oxide Nanoparticles Coated with Polyvinyl Alcohol/5-Fluorouracil/Zinc-Aluminium-Layered Double Hydroxide for a Simultaneous Drug and Target Delivery System. Polymers 2021, 13, 855. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Bini, B.S.; Manjusha, V. Glycyrrhetinic acid conjugated zein capped aminated mesoporous silica nanoparticle-based dual drug delivery system for liver: A pH-dependent triggered release. J. Mol. Liq. 2021, 340, 116852. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, J.; Bai, J.; Li, X.; Tian, C.; Liu, Z.; Zheng, C.; Clark, A.R.; Cheng, X.; Liao, X.; et al. Dual-Ligand-Functionalized Liposomes Based on Glycyrrhetinic Acid and cRGD for Hepatocellular Carcinoma Targeting and Therapy. Mol. Pharm. 2023, 20, 1951–1963. [Google Scholar] [CrossRef]
- Assali, M.; Kittana, N.; Dayyeh, S.; Khiar, N. Dual covalent functionalization of single-walled carbon nanotubes for effective targeted cancer therapy. Nanotechnology 2021, 32, 205101. [Google Scholar] [CrossRef]
- Chen, M.; Xu, X.; Shu, G.; Lu, C.; Wu, J.; Lv, X.; Song, J.; Wu, F.; Chen, C.; Zhang, N.; et al. Multifunctional Microspheres Dual-Loaded with Doxorubicin and Sodium Bicarbonate Nanoparticles to Introduce Synergistic Trimodal Interventional Therapy. Acs Appl. Bio Mater. 2021, 4, 3476–3489. [Google Scholar] [CrossRef]
- Jedrzak, A.; Grzeskowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrowczynski, R. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surf. B-Biointerfaces 2019, 173, 698–708. [Google Scholar] [CrossRef]
- Pandey, B.; Patil, N.G.; Bhosle, G.S.; Arnbade, A.V.; Sen Gupta, S. Amphiphilic Glycopolypeptide Star Copolymer-Based Cross-Linked Nanocarriers for Targeted and Dual-Stimuli-Responsive Drug Delivery. Bioconjugate Chem. 2019, 30, 633–646. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, C.; Zhao, Q.; Chu, Z.; Yang, D.-P.; Jia, N. Sonochemical assisted synthesis of dual functional BSA nanoparticle for the removal of excessive bilirubin and strong anti-tumor effects. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 688–696. [Google Scholar] [CrossRef]
- Pan, X.; Chen, J.; Yang, M.; Wu, J.; He, G.; Yin, Y.; He, M.; Xu, W.; Xu, P.; Cai, W.; et al. Enzyme/pH dual-responsive polymer prodrug nanoparticles based on 10-hydroxycamptothecin-carboxymethylchitosan for enhanced drug stability and anticancer efficacy. Eur. Polym. J. 2019, 117, 372–381. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, C.; Chan, W.; Liu, K.; Lu, A.; Lin, G.; Hu, R.; Shi, H.; Zhang, H.; Yang, Z. Dual-Functional Liposomes with Carbonic Anhydrase IX Antibody and BR2 Peptide Modification Effectively Improve Intracellular Delivery of Cantharidin to Treat Orthotopic Hepatocellular Carcinoma Mice. Molecules 2019, 24, 3332. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneem, M.A.; Elnaggar, M.A.; Hammady, R.S.; Kamel, S.M.; Helmy, M.W.; Abdulkader, M.A.; Zaky, A.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Dual-Targeted Lactoferrin Shell-Oily Core Nanocapsules for Synergistic Targeted/Herbal Therapy of Hepatocellular Carcinoma. Acs Appl. Mater. Interfaces 2019, 11, 26731–26744. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, C.S.; Banerjee, M.; Gupta, S. A Dual Drug Delivery Platform for Cancer-Bacteria Cotargeting. Acs Appl. Bio Mater. 2019, 2, 5032–5041. [Google Scholar] [CrossRef] [PubMed]
- Bullo, S.; Buskaran, K.; Baby, R.; Dorniani, D.; Fakurazi, S.; Hussein, M.Z. Dual Drugs Anticancer Nanoformulation using Graphene Oxide-PEG as Nanocarrier for Protocatechuic Acid and Chlorogenic Acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Ni, W.; Li, Z.; Liu, Z.; Ji, Y.; Wu, L.; Sun, S.; Jian, X.; Gao, X. Dual-Targeting Nanoparticles: Codelivery of Curcumin and 5-Fluorouracil for Synergistic Treatment of Hepatocarcinoma. J. Pharm. Sci. 2019, 108, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhang, Q.; Kuang, G.; Xia, J.; Shang, L. Multicomponent microspheres with spatiotemporal drug release for post-surgical liver cancer treatment and liver regeneration. Chem. Eng. J. 2023, 455, 140585. [Google Scholar] [CrossRef]
- Espinoza, M.J.C.; Lin, K.-S.; Weng, M.-T.; Kunene, S.C.; Lin, Y.-S.; Lin, Y.-T. Synthesis and characterization of silica nanoparticles from rice ashes coated with chitosan/cancer cell membrane for hepatocellular cancer treatment. Int. J. Biol. Macromol. 2023, 228, 487–497. [Google Scholar] [CrossRef] [PubMed]






| Type of Theranostic Nanomedicine | Material (s) | Therapeutic Agent | Diagnostic Agent | Size | Targeting Agent | Advancement |
|---|---|---|---|---|---|---|
| Drug-polymer conjugates | HPMA | 64Cu | 64Cu | N.A. | RGD | Cancer imaging and radiochemo-therapy |
| Polymeric nanoparticles | PLA-TPGS | Docetaxel | Quantum dots | ~250 nm | Folic acid | Co-delivery of docetaxel and quantum dots |
| Solid lipid nanoparticles | Low-density lipoprotein, Cholesterol | Paclitaxel/ siRNA | Quantum dots | ~130 nm | cRGD | Multimodal therapy |
| Dendrimers | Polypropyleni-mine | Phthalocyanines | Phthalo cyanines | ~62 nm | LHRH | Delivery of single theranostic agent |
| Liposomes | TPGS, Phospholipids, Cholesterol | Docetaxel | Quantum dots | ~210 nm | Folic acid | Co-delivery of docetaxel and quantum dots |
| Micelles | TPGS | Iron oxide nanoparticles | Iron oxide nanoparticles | ~178 nm | Passive | Delivery of single theranostic agent |
| Gold nanoparticles | Gold nanoparticles | DOX | Gold nanoparticles | ~55 nm | CPLGLAGG peptide | Stimulus responsive drug release |
| Carbon nanomaterials | SWCNTs | Intrinsic property | Intrinsic property | Length of ~140 nm | Passive | Self-photoluminescent and photothermal property |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Q.; Du, L.; Zhu, L.; Yu, D. Review of the Application of Dual Drug Delivery Nanotheranostic Agents in the Diagnosis and Treatment of Liver Cancer. Molecules 2023, 28, 7004. https://doi.org/10.3390/molecules28207004
Han Q, Du L, Zhu L, Yu D. Review of the Application of Dual Drug Delivery Nanotheranostic Agents in the Diagnosis and Treatment of Liver Cancer. Molecules. 2023; 28(20):7004. https://doi.org/10.3390/molecules28207004
Chicago/Turabian StyleHan, Qinghe, Lianze Du, Lili Zhu, and Duo Yu. 2023. "Review of the Application of Dual Drug Delivery Nanotheranostic Agents in the Diagnosis and Treatment of Liver Cancer" Molecules 28, no. 20: 7004. https://doi.org/10.3390/molecules28207004