The Use of Iron-Doped Anatase TiO2 Nanofibers for Enhanced Photocatalytic Fenton-like Reaction to Degrade Tylosin
Abstract
:1. Introduction
2. Results and Discussion
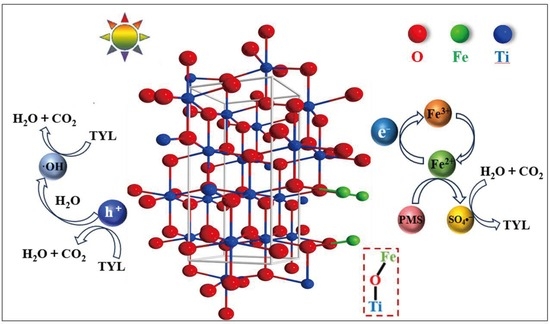
2.1. Structural and Morphological Characterization
2.2. Tylosin Degradation Performance via Photocatalytic Fenton-like Reactions
3. Materials and Methods
3.1. Synthesis of Fe-TNs
3.2. Structural Characterization
3.3. Photocatalytic Fenton-like Degradation for TYL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, C.; Wang, H.; Wang, C.; Zhang, X.; Zhao, X.; Dong, C.-L.; Huang, Y.-C.; Chen, S.; Guo, P.; She, X.; et al. Cooperation of oxygen vacancy and FeIII/FeII sites in H2-reduced Fe-MIL-101 for enhanced Fenton-like degradation of organic pollutants. J. Hazard. Mater. 2023, 441, 129922. [Google Scholar]
- Wang, C.; Wang, X.; Wang, H.; Zhang, L.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Guo, P.; Cai, R.; Haigh, S.J.; et al. Low-coordinated Co-N3 sites induce peroxymonosulfate activation for norfloxacin degradation via high-valent cobalt-oxo species and electron transfer. J. Hazard. Mater. 2023, 455, 131622. [Google Scholar] [PubMed]
- Pang, S.; Zhou, C.; Sun, Y.; Zhang, K.; Ye, W.; Zhao, X.; Cai, L.; Hui, B. Natural wood-derived charcoal embedded with bimetallic iron/cobalt sites to promote ciprofloxacin degradation. J. Clean. Prod. 2023, 414, 137569. [Google Scholar]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic acid/Fe@Fe2O3: A highly efficient combined Fenton reagent to remove organic contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [PubMed]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar]
- Weng, X.; Owens, G.; Chen, Z. Synergetic adsorption and Fenton-like oxidation for simultaneous removal of ofloxacin and enrofloxacin using green synthesized Fe NPs. Chem. Eng. J. 2020, 382, 122871. [Google Scholar]
- Dong, H.; Zou, Y.; Zhang, K.; Sun, Y.; Hui, B.; Yang, D.; Cai, L.; Li, J. Biomimetic design of wood carbon-based heterogeneous catalysts for enhanced organic pollutants degradation. Chem. Eng. J. 2023, 451, 138568. [Google Scholar]
- Cai, H.; Li, X.; Ma, D.; Feng, Q.; Wang, D.; Liu, Z.; Wei, X.; Chen, K.; Lin, H.; Qin, S.; et al. Stable Fe3O4 submicrospheres with SiO2 coating for heterogeneous Fenton-like reaction at alkaline condition. Sci. Total Environ. 2021, 764, 144200. [Google Scholar]
- Zhang, X.; Liu, Y.; Zhai, Y.; Yu, Y.; Guo, Y.; Hao, S. An optimization strategy for photo-Fenton-like catalysts: Based on crystal plane engineering of BiVO4 and electron transfer properties of 0D CQDs. Environ. Res. 2023, 222, 115347. [Google Scholar]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [PubMed]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar]
- Xu, Y.; Zhang, Y.; Wang, X.; Wang, Z.; Huang, L.; Wu, H.; Ren, J.; Gu, C.; Chen, Z. Enhanced photodegradation of tylosin in the presence of natural montmorillonite: Synergistic effects of adsorption and surface hydroxyl radicals. Sci. Total Environ. 2023, 855, 158750. [Google Scholar]
- Zhang, Q.; Li, Y.; Ma, W.; Bai, X.; Ru, X.; Zhang, L.; Zhong, S.; Shu, X. Three-dimensional recyclable FeS2/reduced graphene oxide aerogel with high porosity reticulated structure for efficient removal of tylosin tartrate. Sep. Purif. Technol. 2023, 324, 124463. [Google Scholar]
- Lee, S.; Bayarkhuu, B.; Han, Y.; Kim, H.-W.; Jeong, S.; Boo, C.; Byun, J. Multifunctional photo-Fenton-active membrane for solar-driven water purification. J. Membr. Sci. 2022, 660, 120832. [Google Scholar]
- Dong, S.; Chen, X.; Su, L.; Wen, Y.; Wang, Y.; Yang, Q.; Yi, L.; Xu, W.; Yang, Q.; He, P.; et al. Integration of atomically dispersed Cu-N4 sites with C3N4 for enhanced photo-Fenton degradation over a nonradical mechanism. ACS EST Eng. 2023, 3, 150–164. [Google Scholar]
- Wang, A.; Zheng, Z.; Wang, H.; Chen, Y.; Luo, C.; Liang, D.; Hu, B.; Qiu, R.; Yan, K. 3D hierarchical H2-reduced Mn-doped CeO2 microflowers assembled from nanotubes as a high-performance Fenton-like photocatalyst for tetracycline antibiotics degradation. Appl. Catal. B Environ. 2020, 277, 119171. [Google Scholar]
- Yin, R.; Chen, Y.; He, S.; Li, W.; Zeng, L.; Guo, W.; Zhu, M. In situ photoreduction of structural Fe(III) in a metal–organic framework for peroxydisulfate activation and efficient removal of antibiotics in real wastewater. J. Hazard. Mater. 2020, 388, 121996. [Google Scholar]
- Zhang, L.; Zhang, Q.; Xie, H.; Guo, J.; Lyu, H.; Li, Y.; Sun, Z.; Wang, H.; Guo, Z. Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis. Appl. Catal. B Environ. 2017, 201, 470–478. [Google Scholar]
- Zhao, P.; Yang, Y.; Pei, Y.; Luo, X. TEMPO-oxidized cellulose beads embedded with Au-doped TiO2 nanoparticles for photocatalytic degradation of Tylosin. Cellulose 2023, 30, 1133–1147. [Google Scholar]
- Li, K.; Zhang, S.; Li, Y.; Fan, J.; Lv, K. MXenes as noble-metal-alternative co-catalysts in photocatalysis. Chin. J. Catal. 2021, 42, 3–14. [Google Scholar]
- Liu, C.; Feng, Y.; Han, Z.; Sun, Y.; Wang, X.; Zhang, Q.; Zou, Z. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visi-ble-light-driven photocatalytic activity for organic pollutant removal and hydrogen production. Chin. J. Catal. 2021, 42, 164–174. [Google Scholar]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-atom catalysts based on the metal-oxide interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar]
- Cheng, C.; Ren, W.; Miao, F.; Chen, X.; Chen, X.; Zhang, H. Generation of FeIV=O and its contribution to Fenton-like reactionson a single-atom iron NC catalyst. Angew. Chem. Int. Ed. 2023, 62, e2022185. [Google Scholar]
- Zhang, L.-S.; Jiang, X.-H.; Zhong, Z.-A.; Tian, L.; Sun, Q.; Cui, Y.-T.; Lu, X.; Zou, J.-P.; Luo, S.-L. Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100% selectivity. Angew. Chem. Int. Ed. 2021, 60, 21751–21755. [Google Scholar]
- Li, Y.; Chen, J.; Ji, Y.; Zhao, Z.; Cui, W.; Sang, X.; Cheng, Y.; Yang, B.; Li, Z.; Zhang, Q.; et al. Single-atom iron catalyst with biomimetic active center to accelerate proton spillover for medical-level elec-trosynthesis of H2O2 disinfectant. Angew. Chem. Int. Ed. 2023, 62, e202306491. [Google Scholar]
- Xu, X.; Zhan, F.; Pan, J.; Zhou, L.; Su, L.; Cen, W.; Li, W.; Tian, C. Engineering single-atom Fe-Pyridine N4 sites to boost peroxymonosulfate activation for antibiotic degradation in a wide pH range. Chemosphere 2022, 294, 133735. [Google Scholar]
- Song, W.; Xiao, X.; Wang, G.; Dong, X.; Zhang, X. Highly efficient peroxymonosulfate activation on Fe-N-C catalyst via the collaboration of low-coordinated Fe-N structure and Fe nanoparticles for enhanced organic pollutant degradation. J. Hazard. Mater. 2023, 455, 131596. [Google Scholar]
- Wang, P.; Liu, X.; Qiu, W.; Wang, F.; Jiang, H.; Chen, M.; Zhang, W.; Ma, J. Catalytic degradation of micropollutant by peroxymonosulfate activation through Fe(III)/Fe(II) cycle confined in the nanoscale interlayer of Fe(III)-saturated montmorillonite. Water Res. 2020, 182, 116030. [Google Scholar]
- Yin, K.; Peng, L.; Chen, D.; Liu, S.; Zhang, Y.; Gao, B.; Fu, K.; Shang, Y.; Xu, X. High-loading of well dispersed single-atom catalysts derived from Fe-rich marine algae for boosting Fenton-like reaction: Role identification of iron center and catalytic mechanisms. Appl. Catal. B Environ. 2023, 336, 122951. [Google Scholar]
- An, S.F.; Zhang, G.H.; Wang, T.W.; Zhan, W.N.; Li, K.Y.; Song, C.S.; Miller, J.T.; Miao, S.; Wang, J.H.; Guo, X.W. High-density ultra-small clusters and single-atom Fe sites embedded in graphitic carbon nitride (g-C3N4) for highly efficient catalytic advanced oxidation processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar]
- Liu, Y.; Wang, X.; Ye, W.; Butenko, D.S.; Lu, P.; Chen, Q.; Cai, R.; Sun, J.; Zhu, Y.; Yang, D. FeOx nanoclusters decorated TiO2 for boosting white LED driven photocatalytic Fenton-like norfloxacin degradation. Sep. Purif. Technol. 2022, 303, 122194. [Google Scholar]
- Ling, F.; Xiao, X.; Li, Y.; Li, W. A Zn/Co bimetal zeolitic imidazolate framework material as a catalyst to activate persulfates to degrade tylosin in aqueous solutions. New J. Chem. 2022, 46, 18917–18925. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, G.; Yang, C.; Chen, S.; Xu, Y.; Zhang, S.; Lu, P.; Sun, J.; Zhu, Y.; Yang, D. Efficient photoelectrocatalytic degradation of tylosin on TiO2 nanotube arrays with tunable phosphorus dopants. J. Environ. Chem. Eng. 2021, 9, 104742. [Google Scholar]
- Tizhoosh, L.Y.; Khataee, A.; Hassandoost, R.; Soltani, R.D.C.; Doustkhah, E. Ultrasound-engineered synthesis of WS2@CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochem. 2020, 67, 105114. [Google Scholar]
- Tong, Y.-H.; Wu, Y.-Z.; Xu, Z.-L.; Luo, L.-H.; Xu, S.-J. Photocatalytic self-cleaning EVAL membrane by incorporating bio-inspired functionalized MIL-101(Fe) for dye/salt separation. Chem. Eng. J. 2022, 444, 136507. [Google Scholar]
- Wang, X.; Ma, Y.; Jiang, J.; Li, M.; Li, T.; Li, C.; Dong, S. Cl-based functional group modification MIL-53(Fe) as efficient photocatalysts for degradation of tetracycline hydrochloride. J. Hazard. Mater. 2022, 434, 128864. [Google Scholar] [CrossRef]
- Jiang, Y.; Ran, J.; Mao, K.; Yang, X.; Zhong, L.; Yang, C.; Feng, X.; Zhang, H. Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicol. Environ. Saf. 2022, 236, 113464. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lu, W.; Zhang, S.; Guo, C.; Yang, K.; Sun, Y.; Shao, Y.; Li, Q.; Bu, M.; Wu, L.; et al. The Use of Iron-Doped Anatase TiO2 Nanofibers for Enhanced Photocatalytic Fenton-like Reaction to Degrade Tylosin. Molecules 2023, 28, 6977. https://doi.org/10.3390/molecules28196977
Wang X, Lu W, Zhang S, Guo C, Yang K, Sun Y, Shao Y, Li Q, Bu M, Wu L, et al. The Use of Iron-Doped Anatase TiO2 Nanofibers for Enhanced Photocatalytic Fenton-like Reaction to Degrade Tylosin. Molecules. 2023; 28(19):6977. https://doi.org/10.3390/molecules28196977
Chicago/Turabian StyleWang, Xiao, Wei Lu, Shangui Zhang, Changqing Guo, Kai Yang, Yan Sun, Yashi Shao, Qiyuan Li, Mingsheng Bu, Lianfeng Wu, and et al. 2023. "The Use of Iron-Doped Anatase TiO2 Nanofibers for Enhanced Photocatalytic Fenton-like Reaction to Degrade Tylosin" Molecules 28, no. 19: 6977. https://doi.org/10.3390/molecules28196977