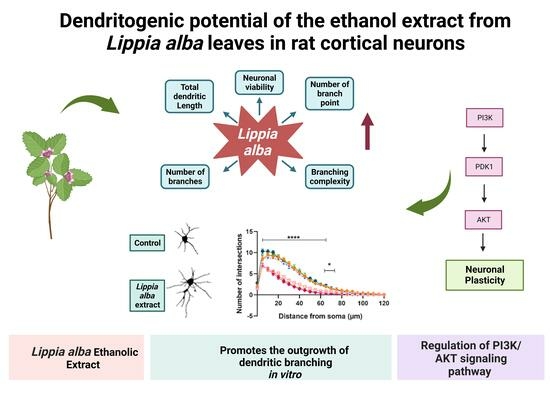
Dendritogenic Potential of the Ethanol Extract from Lippia alba Leaves in Rat Cortical Neurons
Abstract
:1. Introduction
2. Results
2.1. Analysis of Secondary Metabolites in Lippia alba Leaves Extract
2.2. Lippia alba Extract Increases the Dendritic Complexity of Cortical Neurons
2.3. Specific Inhibitors of ERK1/ERK2 and AKT Do Not Affect the Viability of Neurons after 6 h of Treatment
2.4. PI3K Pathway Is Involved in the Dendritogenic Effect of Lippia alba Extract on Cortical Neurons
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extract Preparation
4.2. Chemical Composition Analysis
4.3. Animals
4.4. Primary Culture of Cortical Neurons
4.5. Effect of Lippia alba Extract and Specific Inhibitors on the Dendritic Complexity of Cortical Neurons
4.5.1. Treatment with Lippia alba Extract and/or Specific Inhibitors
4.5.2. Immunohistochemistry
4.5.3. Imaging and Processing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
References
- Lefebvre, J.L.; Sanes, J.R.; Kay, J.N.; Sickkids, J.L. Development of Dendritic Form and Function. Annu. Rev. Cell. Dev. Biol. 2015, 31, 741–777. [Google Scholar] [CrossRef]
- Von Bohlen Und Halbach, O. Analysis of Morphological Changes as a Key Method in Studying Psychiatric Animal Models. Cell. Tissue Res. 2013, 354, 41–50. [Google Scholar] [CrossRef]
- O’Neill, K.M.; Akum, B.F.; Dhawan, S.T.; Kwon, M.; Langhammer, C.G.; Firestein, B.L. Assessing Effects on Dendritic Arborization Using Novel Sholl Analyses. Front. Cell. Neurosci. 2015, 9, 285. [Google Scholar] [CrossRef]
- Kulkarni, V.A.; Firestein, B.L. The Dendritic Tree and Brain Disorders. Mol. Cell. Neurosci. 2012, 50, 10–20. [Google Scholar] [CrossRef]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic Structural Plasticity and Neuropsychiatric Disease. Nat. Rev. Neurosci. 2018, 19, 215–234. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Nikonenko, I.; Muller, D. Structural Plasticity: Mechanisms and Contribution to Developmental Psychiatric Disorders. Front. Neuroanat. 2014, 8, 123. [Google Scholar] [CrossRef]
- Quach, T.T.; Stratton, H.J.; Khanna, R.; Kolattukudy, P.E.; Honnorat, J.; Meyer, K.; Duchemin, A.M. Intellectual Disability: Dendritic Anomalies and Emerging Genetic Perspectives. Acta Neuropathol. 2020, 141, 139–158. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural. Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef]
- Colyn, L.; Venzala, E.; Marco, S.; Perez-Otaño, I.; Tordera, R.M. Chronic Social Defeat Stress Induces Sustained Synaptic Structural Changes in the Prefrontal Cortex and Amygdala. Behav. Brain Res. 2019, 373, 112079. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Wang, X.-L.; Feng, S.-T.; Chen, N.-H.; Wang, Z.-Z.; Zhang, Y. Novel Rapid-Acting Glutamatergic Modulators: Targeting the Synaptic Plasticity in Depression. Pharmacol. Res. 2021, 171, 1043–6618. [Google Scholar] [CrossRef]
- Culpepper, L.; Lam, R.W.; McIntyre, R.S. Cognitive Impairment in Patients with Depression: Awareness, Assessment, and Management. J. Clin. Psychiatry 2017, 78, 1383–1394. [Google Scholar] [CrossRef]
- Rădulescu, I.; Drăgoi, A.M.; Trifu, S.C.; Cristea, M.B. Neuroplasticity and Depression: Rewiring the Brain’s Networks through Pharmacological Therapy (Review). Exp. Ther. Med. 2021, 22, 1131. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 500839. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and Molecular Mechanisms Regulating Neuronal Growth by Brain-Derived Neurotrophic Factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lane, H.Y.; Lin, C.H. New Treatment Strategies of Depression: Based on Mechanisms Related to Neuroplasticity. Neural. Plast. 2017, 2017, 4605971. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal Cortex and Depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef]
- Belleau, E.L.; Treadway, M.T.; Pizzagalli, D.A. The Impact of Stress and Major Depressive Disorder on Hippocampal and Medial Prefrontal Cortex Morphology. Biol. Psychiatry 2019, 85, 443–453. [Google Scholar] [CrossRef]
- Johnson, M.W.; Griffiths, R.R. Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 2017, 14, 734–740. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Roseman, L.; Bolstridge, M.; Demetriou, L.; Pannekoek, J.N.; Wall, M.B.; Tanner, M.; Kaelen, M.; McGonigle, J.; Murphy, K.; et al. Psilocybin for Treatment-Resistant Depression: FMRI-Measured Brain Mechanisms. Sci. Rep. 2017, 7, 13187. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell. Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Lee, H.M.; Roth, B.L. Hallucinogen Actions on Human Brain Revealed. Proc. Natl. Acad. Sci. USA 2012, 109, 1820–1821. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.V.; Meyer, R.; Avanes, A.A.; Rus, M.; Olson, D.E. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry 2021, 12, 727117. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Ross, S. Therapeutic Applications of Classic Hallucinogens. Curr. Top. Behav. Neurosci. 2018, 36, 361–391. [Google Scholar] [CrossRef]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- de Veen, B.T.H.; Schellekens, A.F.A.; Verheij, M.M.M.; Homberg, J.R. Psilocybin for Treating Substance Use Disorders? Expert Rev. Neurother. 2017, 17, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Griffiths, R.R.; Hendricks, P.S.; Henningfield, J.E. The Abuse Potential of Medical Psilocybin According to the 8 Factors of the Controlled Substances Act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Nutt, D.J.; King, L.A.; Nichols, D.E. Effects of Schedule I Drug Laws on Neuroscience Research and Treatment Innovation. Nat. Rev. Neurosci. 2013, 14, 577–585. [Google Scholar] [CrossRef]
- Alvarado-García, P.A.; Soto-Vásquez, M.R.; Rosales-Cerquin, L.E.; Alfaro-Ttito, B.M.; Rodrigo-Villanueva, E.M. Anxiolytic-like Effect of Essential Oils Extracted from Lippia alba and Lippia citriodora. Pharmacogn. J. 2021, 13, 1377–1383. [Google Scholar] [CrossRef]
- Da Silva, L.V.F.; Mouraõ, R.H.V.; Manimala, J.; Lnenicka, G.A. The Essential Oil of Lippia alba and Its Components Affect Drosophila Behavior and Synaptic Physiology. J. Exp. Biol. 2018, 221, jeb176909. [Google Scholar] [CrossRef]
- Serafini, G. Neuroplasticity and Major Depression, the Role of Modern Antidepressant Drugs. World J. Psychiatry 2012, 2, 49. [Google Scholar] [CrossRef]
- McEwen, B.S.; Eiland, L.; Hunter, R.G.; Miller, M.M. Stress and Anxiety: Structural Plasticity and Epigenetic Regulation as a Consequence of Stress. Neuropharmacology 2012, 62, 3–12. [Google Scholar] [CrossRef]
- Copf, T. Impairments in Dendrite Morphogenesis as Etiology for Neurodevelopmental Disorders and Implications for Therapeutic Treatments. Neurosci. Biobehav. Rev. 2016, 68, 946–978. [Google Scholar] [CrossRef]
- Jan, Y.N.; Jan, L.Y. Branching out: Mechanisms of Dendritic Arborization. Nat. Rev. Neurosci. 2010, 11, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, J.; Yu, B.; Ba, R.; Zhao, C. Brpf1 Haploinsufficiency Impairs Dendritic Arborization and Spine Formation, Leading to Cognitive Deficits. Front. Cell Neurosci. 2019, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.L.G.; Chilpa, R.R.; Jaime, H.B. Medicinal Plants for the Treatment of “Nervios”, Anxiety, and Depression in Mexican Traditional Medicine. Rev. Bras. Farmacogn. 2014, 24, 591–608. [Google Scholar] [CrossRef]
- Siqueira-Lima, P.S.; Passos, F.R.S.; Lucchese, A.M.; Menezes, I.R.A.; Coutinho, H.D.M.; Lima, A.A.N.; Zengin, G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Central Nervous System and Analgesic Profiles of Lippia Genus. Rev. Bras. Farmacogn. 2019, 29, 125–135. [Google Scholar] [CrossRef]
- Parente, M.S.R.; Custódio, F.R.; Cardoso, N.A.; Lima, M.J.A.; Melo, T.; Linhares, M.I.; Siqueira, R.M.P.; Nascimento, A.; Catunda Júnior, F.E.A.; Melo, C.; et al. Antidepressant-like Effect of Lippia sidoides CHAM (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018, 86, 27. [Google Scholar] [CrossRef]
- Haldar, S.; Kar, B.; Dolai, N.; Kumar, R.B.S.; Behera, B.; Haldar, P.K. In Vivo Anti-Nociceptive and Anti-Inflammatory Activities of Lippia alba. Asian Pac. J. Trop. Dis. 2012, 2, S667–S670. [Google Scholar] [CrossRef]
- Do Vale, T.G.; Furtado, E.C.; Santos, J.G.; Viana, G.S.B. Central Effects of Citral, Myrcene and Limonene, Constituents of Essential Oil Chemotypes from Lippia alba (Mill.) N.E. Brown. Phytomedicine 2002, 9, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Montiel-Ramos, J.; Zapata, B.; Durán, C.; Betancur-Galvis, L.; Stashenko, E. Citral and Carvone Chemotypes from the Essential Oils of Colombian Lippia alba (Mill.) N.E. Brown: Composition, Cytotoxicity and Antifungal Activity. Mem. Inst. Oswaldo Cruz. 2009, 104, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, T.; Sahpaz, S.; Gressier, B.; Joseph, H.; Bailleul, F. Antioxidant and Neurosedative Properties of Polyphenols and Iridoids from Lippia alba. Phytother. Res. 2008, 22, 256–258. [Google Scholar] [CrossRef]
- Chies, C.E.; Branco, C.S.; Scola, G.; Agostini, F.; Gower, A.E.; Salvador, M. Antioxidant Effect of Lippia alba (Miller) N. E. Brown. Antioxidants 2013, 2, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant Terpenes: Defense Responses, Phylogenetic Analysis, Regulation and Clinical Applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of Terpenes in Cyclodextrins: Preparation, Characterization and Pharmacological Approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Fraga, C.G. Research Trends in Flavonoids and Health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Stotz, S.C.; Vriens, J.; Martyn, D.; Clardy, J.; Clapham, D.E. Correction: Citral Sensing by Transient Receptor Potential Channels in Dorsal Root Ganglion Neurons. PLoS ONE 2008, 3, e2082. [Google Scholar] [CrossRef]
- Quintans, L.J.; Guimarães, A.G.; de Santana, M.T.; Araújo, B.E.S.; Moreira, F.V.; Bonjardim, L.R.; Araújo, A.A.S.; Siqueira, J.S.; Ângelo, A.R.; Botelho, M.A.; et al. Citral Reduces Nociceptive and Inflammatory Response in Rodents. Rev. Bras. Farmacogn. 2011, 21, 497–502. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of Hippocampal Plasticity and Cognitive Behavior by Short-Term Blueberry Supplementation in Aged Rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Merino, C.; Lopez-Sanchez, C.; Lagoa, R.; Samhan-Arias, A.K.; Bueno, C.; Garcia-Martinez, V. Neuroprotective Actions of Flavonoids. Curr. Med. Chem. 2011, 18, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. The Impact of Flavonoids on Memory: Physiological and Molecular Considerations. Chem. Soc. Rev. 2009, 38, 1152–1161. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in Modulation of Cell Survival Signalling Pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of Neurotrophic Signaling Pathways by Polyphenols. Drug Des. Devel. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef]
- Mullen, L.M.; Pak, K.K.; Chavez, E.; Kondo, K.; Brand, Y.; Ryan, A.F. Ras/P38 and PI3K/Akt but Not Mek/Erk Signaling Mediate BDNF-Induced Neurite Formation on Neonatal Cochlear Spiral Ganglion Explants. Brain Res. 2012, 1430, 25–34. [Google Scholar] [CrossRef]
- Jin, Y.; Sui, H.J.; Dong, Y.; Ding, Q.; Qu, W.H.; Yu, S.X.; Jin, Y.X. Atorvastatin Enhances Neurite Outgrowth in Cortical Neurons in Vitro via Up-Regulating the Akt/MTOR and Akt/GSK-3β Signaling Pathways. Acta Pharmacol. Sin. 2012, 33, 861–872. [Google Scholar] [CrossRef]
- Kumar, V.; Zhang, M.-X.X.; Swank, M.W.; Kunz, J.; Wu, G.-Y.Y. Regulation of Dendritic Morphogenesis by Ras-PI3K-Akt-MTOR and Ras-MAPK Signaling Pathways. J. Neurosci. 2005, 25, 11288–11299. [Google Scholar] [CrossRef]
- Nourbakhsh, K.; Yadav, S. Kinase Signaling in Dendritic Development and Disease. Front. Cell. Neurosci. 2021, 15, 624648. [Google Scholar] [CrossRef]
- Sanabria-Galindo, A.; López, S.I.; Gualdrón, R. Estudio Fitoquímico Preliminar y Letalidad Sobre Artemia Salina de Plantas Colombianas. Rev. Colomb. Cienc. Quím. Farm. 1997, 26, 15–19. [Google Scholar]
- Beaudoin, G.M.J.; Lee, S.H.; Singh, D.; Yuan, Y.; Ng, Y.G.; Reichardt, L.F.; Arikkath, J. Culturing Pyramidal Neurons from the Early Postnatal Mouse Hippocampus and Cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A. Evaluación Del Efecto Neuroprotector y de Los Cambios en la Complejidad Dendrítica Inducidos por los Extractos de Tillandsia usneoides y Lippia alba en Cultivo Primario de Neuronas Tratadas Con Agentes Quimioterapéuticos. Master’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, 2021. [Google Scholar]
- Gerlier, D.; Thomasset, N. Use of MTT Colorimetric Assay to Measure Cell Activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]
- Korzhevskii, D.E.; Karpenko, M.N.; Kirik, O.V. Microtubule-Associated Proteins as Indicators of Differentiation and the Functional State of Nerve Cells. Neurosci. Behav. Physiol. 2012, 42, 215–222. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rishal, I.; Golani, O.; Rajman, M.; Costa, B.; Ben-Yaakov, K.; Schoenmann, Z.; Yaron, A.; Basri, R.; Fainzilber, M.; Galun, M. WIS-Neuromath Enables Versatile High Throughput Analyses of Neuronal Processes. Dev. Neurobiol. 2012, 73, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.A. Dendritic Organization in the Neurons of the Visual and Motor Cortices of the Cat. J. Anat. 1953, 87, 387. [Google Scholar] [PubMed]
- Uylings, H.B.M.; Pelt, J. van Measures for Quantifying Dendritic Arborizations. Netw. Comput. Neural Syst. 2002, 13, 397. [Google Scholar] [CrossRef]
- Ristanović, D.; Milošević, N.T.; Štulić, V. Application of Modified Sholl Analysis to Neuronal Dendritic Arborization of the Cat Spinal Cord. J. Neurosci. Methods 2006, 158, 212–218. [Google Scholar] [CrossRef]
- Schmitz, S.K.; Hjorth, J.J.J.J.; Joemai, R.M.S.S.; Wijntjes, R.; Eijgenraam, S.; de Bruijn, P.; Georgiou, C.; de Jong, A.P.H.H.; van Ooyen, A.; Verhage, M.; et al. Automated Analysis of Neuronal Morphology, Synapse Number and Synaptic Recruitment. J. Neurosci. Methods 2011, 195, 185–193. [Google Scholar] [CrossRef]






| Metabolite Family | Chemical Test | Positive Control | Observation |
|---|---|---|---|
| Steroids | Vanillin-H2SO4 | Lupeol | Negative |
| Flavonoids | Shinoda | Kaempferol | Positive |
| Phenolic compounds | FeCl3 1% | Tannic acid | Positive |
| Coumarins | Fluorescence + NaOH | Coumarin | Negative |
| Alkaloids | Dragendorff | Theobromine | Negative |
| Tannins | Lead acetate 10% | Gallic acid | Positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez, M.M.; Lattig, M.C.; Chitiva, L.C.; Costa, G.M.; Sutachan, J.J.; Albarracin, S.L. Dendritogenic Potential of the Ethanol Extract from Lippia alba Leaves in Rat Cortical Neurons. Molecules 2023, 28, 6666. https://doi.org/10.3390/molecules28186666
Velásquez MM, Lattig MC, Chitiva LC, Costa GM, Sutachan JJ, Albarracin SL. Dendritogenic Potential of the Ethanol Extract from Lippia alba Leaves in Rat Cortical Neurons. Molecules. 2023; 28(18):6666. https://doi.org/10.3390/molecules28186666
Chicago/Turabian StyleVelásquez, María Marcela, María Claudia Lattig, Luis Carlos Chitiva, Geison M. Costa, Jhon Jairo Sutachan, and Sonia Luz Albarracin. 2023. "Dendritogenic Potential of the Ethanol Extract from Lippia alba Leaves in Rat Cortical Neurons" Molecules 28, no. 18: 6666. https://doi.org/10.3390/molecules28186666