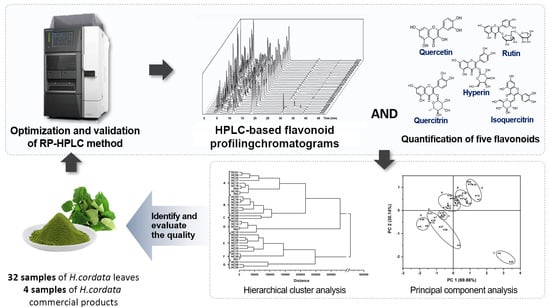
RP-HPLC-Based Flavonoid Profiling Accompanied with Multivariate Analysis: An Efficient Approach for Quality Assessment of Houttuynia cordata Thunb Leaves and Their Commercial Products
Abstract
:1. Introduction
2. Results
2.1. Optimization of RP-HPLC Conditions for Flavonoid Analysis
2.2. Validation of Optimized RP-HPLC Method
2.2.1. Specificity
2.2.2. Precision, Repeatability, Intermediate Precision, and Stability
2.2.3. Accuracy
2.2.4. Linearity, Range, Limit of Detection, and Limit of Quantification
2.3. HPLC-Based Flavonoid Profiles
2.4. Quantification Results of 36 H. cordata Leaves Samples
2.5. Principal Component Analysis (PCA)
2.6. Hierarchical Cluster Analysis (HCA)
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Instrumentations
4.3. Sampling H. cordata Leaves
4.4. Preparation of Flavonoids Standard Solution
4.5. Preparation of Houttuynia Cordata Leaves Sample Solution
4.6. RP-HPLC Method Optimization for the Analysis of the Flavonoids
4.7. RP-HPLC Method Validation
4.7.1. Specificity
4.7.2. Stability
4.7.3. Accuracy
4.7.4. Precision
4.7.5. Linearity and Range
4.7.6. LOD and LOQ
4.8. Quantitative Analysis of Flavonoids Content
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FDA. Botanical Drug Development: Guidance for Industry. Available online: https://www.fda.gov/media/93113/download (accessed on 12 May 2022).
- EMA. Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-herbal-medicinal-products-traditional-herbal-medicinal-products-revision-2_en.pdf (accessed on 12 May 2022).
- WHO. Programme on Traditional Medicine. Guidelines for the Assessment of Herbal Medicines. Available online: https://apps.who.int/iris/bitstream/handle/10665/58865/WHO_TRM_91.4.pdf?sequence=1&isAllowed=y (accessed on 12 May 2022).
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2015.
- Ministry of Health. Vietnamese Pharmacopoeia V; Medical Publshing House: Hanoi, Vietnam, 2017.
- Hawrył, A.; Hawrył, M.; Litwińczuk, W.; Bogucka-Kocka, A. Thin-layer chromatographic fingerprint of selected Paulownia species with chemometrics and antioxidant activity. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 367–374. [Google Scholar] [CrossRef]
- Pedan, V.; Weber, C.; Do, T.; Fischer, N.; Reich, E.; Rohn, S. HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chem. 2018, 267, 277–287. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, T.; Wu, L.; Zhang, J.; Zuo, Z.; Wang, Y. Identification of Gentiana rigescens from different geographical origins based on HPLC and FTIR fingerprints. Anal. Methods 2020, 12, 2260–2271. [Google Scholar] [CrossRef]
- Zhuo, Z.; Xu, D.; Li, Y.; Pu, B.; Ye, M. Fingerprint analysis of Zanthoxylum armatum DC. by HPLC. J. Food Compos. Anal. 2021, 96, 103736. [Google Scholar] [CrossRef]
- Yan, X.; Wang, W.; Chen, Z.; Xie, Y.; Li, Q.; Yu, Z.; Hu, H.; Wang, Z. Quality assessment and differentiation of Aucklandiae Radix and Vladimiriae Radix based on GC-MS fingerprint and chemometrics analysis: Basis for clinical application. Anal. Bioanal. Chem. 2020, 412, 1535–1549. [Google Scholar] [CrossRef]
- Xie, P.; Chen, S.; Liang, Y.-z.; Wang, X.; Tian, R.; Upton, R. Chromatographic fingerprint analysis—A rational approach for quality assessment of traditional Chinese herbal medicine. J. Chromatogr. A 2006, 1112, 171–180. [Google Scholar] [CrossRef]
- Xie, B.; Gong, T.; Tang, M.; Mi, D.; Zhang, X.; Liu, J.; Zhang, Z. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers. J. Pharm. Biomed. Anal. 2008, 48, 1261–1266. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Xi, J.; Pan, C.; Xu, Z.; Xia, W.; Zhang, W. Multiple-fingerprint analysis of Poria cocos polysaccharide by HPLC combined with chemometrics methods. J. Pharm. Biomed. Anal. 2021, 198, 114012. [Google Scholar] [CrossRef]
- Patel, P.R.; Patel, N.D.; Patel, S.G.; Kanaki, N.S.; Patel, A.J. Fingerprint Analysis of Vitex Negundo by HPLC Coupled with Multi-components Analysis. Curr. Pharm. Anal. 2020, 16, 743–751. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.-H.; Zhang, Q.; Lai, M.-X.; Wang, Q. Quality assessment of Cortex cinnamomi by HPLC chemical fingerprint, principle component analysis and cluster analysis. J. Sep. Sci. 2007, 30, 1276–1283. [Google Scholar] [CrossRef]
- Yu, B.; Li, Z.; Wu, J.; Ying, J.; Tang, Y.; Wu, B.; Tang, C.; Xu, J. Quality Control of Gastrodia elata by High-Performance Liquid Chromatography with Fluorescence Detection (HPLC–FLD) and Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA). Anal. Lett. 2020, 53, 746–759. [Google Scholar] [CrossRef]
- Wei, W.; Youliang, Z.; Li, C.; Yuming, W.; Zehong, Y.; Ruiwu, Y. PCR-RFLP analysis of cpDNA and mtDNA in the genus Houttuynia in some areas of China. Hereditas 2005, 142, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Loi, D.T. Vietnamese Medicinal Plants and Remedies; Medical Publishing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Bich, D.H.; Chung, D.Q.; Chuong, B.X.; Dong, N.T.; Dam, D.T.; Hien, P.V.; Lo, V.N.; Mai, P.D.; Man, P.K.; Nhu, D.T.; et al. Plants and Animals Used as Medicine in Vietnam; Science and Technics Publishing House: Hanoi, Vietnam, 2004; Volume 1 & 2. [Google Scholar]
- Chen, A.; Xiang, W.; Liu, D.; Liu, C.; Yang, L. Determination of total flavonoids and its antioxidant ability in Houttuynia cordata. MSCE 2016, 4, 131–136. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Mizuguchi, H.; Amoh, T.; Ogino, S.; Matsuo, T.; Miyake, Y.; Fukui, H.; Kashiwada, Y. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem. 2016, 80, 1205–1213. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Yang, L.P. Systematic analysis of components and contents in Houttuynia cordata Thunb. TMR 2017, 2, 176–188. [Google Scholar] [CrossRef]
- Yang, Z.-n.; Sun, Y.-m.; Luo, S.-q.; Chen, J.-w.; Yu, Z.-w.; Sun, M. Quality evaluation of Houttuynia cordata Thunb. by high performance liquid chromatography with photodiode-array detection (HPLC-DAD). Pak. J. Pharm. Sci. 2014, 27, 223–231. [Google Scholar]
- Qi, S.; Zha, L.; Peng, Y.; Luo, W.; Chen, K.; Li, X.; Huang, D.; Yin, D. Quality and Metabolomics Analysis of Houttuynia cordata Based on HS-SPME/GC-MS. Molecules 2022, 27, 3921. [Google Scholar] [CrossRef]
- Tian, L.; Shi, X.; Yu, L.; Zhu, J.; Ma, R.; Yang, X. Chemical Composition and Hepatoprotective Effects of Polyphenol-Rich Extract from Houttuynia cordata Tea. J. Agric. Food Chem. 2012, 60, 4641–4648. [Google Scholar] [CrossRef]
- Ling, L.-j.; Lu, Y.; Zhang, Y.-y.; Zhu, H.-y.; Tu, P.; Li, H.; Chen, D.-f. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef] [PubMed]
- Ling-Shang, W.; Jin-Ping, S.; Xiao-Qing, Y.; Xue-Rong, S. Quantitive variation of flavonoids in Houttuynia cordata from different geographic origins in China. Chin. J. Nat. Med. 2009, 7, 40–46. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Transactions. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Ramirez-Gomez, X.S.; Beltran-Campos, V.; Contreras-Medina, L.M.; Garcia-Trejo, J.F.; Feregrino-Pérez, A.A. Changes in the Content of Phenolic Compounds and Biological Activity in Traditional Mexican Herbal Infusions with Different Drying Methods. Molecules 2020, 25, 1601. [Google Scholar] [CrossRef] [PubMed]
- Hien, N.M.; Huong, T.T.; Minh, D.B.; Loan, H.T.; Tri, L.M. Application of response surface methodology to optimize the ultrasound-assisted flavonoid-rich extraction of fish mint (Houttuynia cordata Thunb.). Sci. Technol. Dev. J. 2021, 24, 1994–2003. [Google Scholar]
- Teasdale, A.; Elder, D.; Nims, R.W. ICH Quality Guidelines: An Implementation Guide; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]






| Column | Elution Program | Flow Rate (mL min−1) | Column Temp. | Mobile Phase | Peak | Retention Time (min) | Relative Retention Time (min) | Resolution | Tailing Factor | The Number of Theoretical Plates |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenomenex 250 mm × 4.6 mm, 5 μm | Program A: 0–15 min: 20% B; 15–25 min: 20–21% B; 25–40 min: 21–70% B. | 1 | 25 °C | AA 0.2% (A):ACN (B) | 1 | 8.07 | 0.923 | ― | 0.999 | 4813 |
| 2 | 9.75 | 0.892 | 3.652 | ― | 7394 | |||||
| 3 | 10.12 | 0.885 | 0.457 | ― | 1243 | |||||
| 4 | 15.62 | 0.857 | 6.060 | 1.126 | 8756 | |||||
| 5 | 33.06 | 0.860 | 36.027 | 1.879 | 193,035 | |||||
| FA 0.1% (A):ACN (B) | 1 | 8.24 | 0.943 | ― | 1.016 | 4864 | ||||
| 2 | 9.95 | 0.910 | 3.697 | ― | 7746 | |||||
| 3 | 10.33 | 0.904 | 0.555 | ― | 2010 | |||||
| 4 | 16.04 | 0.880 | 7.162 | 1.157 | 9116 | |||||
| 5 | 33.23 | 0.864 | 33.483 | 1.961 | 140,203 | |||||
| Program B: 0–10 min: 20% B; 10–30 min: 20–21% B; 30–50 min: 21–70% B. | 0.9 | 25 °C | AA 0.2% (A):ACN (B) | 1 | 8.98 | 1.027 | ― | 0.997 | 5254 | |
| 2 | 10.84 | 0.992 | 3.809 | ― | 8156 | |||||
| 3 | 11.25 | 0.984 | 0.600 | ― | 2513 | |||||
| 4 | 17.31 | 0.950 | 7.688 | 1.124 | 10377 | |||||
| 5 | 39.03 | 1.015 | 40.597 | 1.297 | 160,129 | |||||
| FA 0.1% (A):ACN (B) | 1 | 9.01 | 1.031 | ― | 1.026 | 5196 | ||||
| 2 | 10.92 | 0.999 | 3.886 | ― | 8315 | |||||
| 3 | 11.34 | 0.992 | 0.645 | ― | 3035 | |||||
| 4 | 17.54 | 0.963 | 8.232 | 1.156 | 10489 | |||||
| 5 | 39.16 | 1.018 | 44.222 | 1.069 | 287,119 | |||||
| Phenomenex 150 mm × 4.6 mm, 5 μm | Program C: 0–5 min: 20% B; 5–25 min: 20–21% B; 25–45 min: 21–50% B. | 0.6 | 25 °C | FA 0.1% (A):ACN (B) | 1 | 7.88 | 0.902 | ― | 0.992 | 7465 |
| 2 | 9.93 | 0.909 | 5.221 | ― | 8870 | |||||
| 3 | 10.35 | 0.906 | 0.909 | ― | 6873 | |||||
| 4 | 16.45 | 0.903 | 10.798 | 0.904 | 10,870 | |||||
| 5 | 37.35 | 0.971 | 42.743 | 1.058 | 185,427 | |||||
| 40 °C | FA 0.1% (A):ACN (B) | 1 | 5.69 | 0.651 | ― | 1.019 | 4905 | |||
| 2 | 7.10 | 0.650 | 4.184 | ― | 6561 | |||||
| 3 | 7.40 | 0.647 | 0.659 | ― | 2850 | |||||
| 4 | 11.71 | 0.643 | 8.329 | 0.867 | 9489 | |||||
| 5 | 33.83 | 0.880 | 59.77 | 0.999 | 272,802 |
| Standards | Precision | Intermediate Precision | Repeatability | Stability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day (n = 6) | Inter-Day (n = 6) | Intra-Day and Inter-Day (n = 12) | (n = 5) | 0, 3, 6, 9, and 12 h | ||||||
| Mean | %RSD | Mean | %RSD | Mean | %RSD | Mean | %RSD | Mean | %RSD | |
| Rutin hydrate | 173,008.3 | 1.76 | 175,346.8 | 1.40 | 174,177.6 | 1.13 | 173,715 | 1.62 | 175,213.2 | 1.45 |
| Hyperin | 300,690.2 | 0.38 | 310,497.3 | 0.89 | 305,593.8 | 1.80 | 300,476 | 0.37 | 305,351.6 | 1.69 |
| Isoquercitrin | 63,249.83 | 1.26 | 667,22.17 | 1.21 | 64,986 | 1.27 | 63,043 | 1.37 | 65,089 | 1.97 |
| Quercitrin | 1,267,000 | 0.25 | 1,308,277 | 0.95 | 1,287,638 | 1.80 | 1,267,369 | 0.27 | 1,290,176 | 1.49 |
| Quercetin | 32,355.83 | 1.14 | 33,940 | 1.48 | 33,147.92 | 1.12 | 32,245 | 0.87 | 32,882 | 2.76 |
| Standards | Added Conc. (µg/mL) | Detected Conc. (µg/mL) | Recovery * (%) |
|---|---|---|---|
| Rutin (1) (73.13 µg/mL) | 58.56 | 131.71 | 100.03 |
| 73.12 | 147.23 | 101.34 | |
| 88.04 | 160.51 | 99.25 | |
| Hyperin (2) (32.06 µg/mL) | 25.64 | 58.14 | 101.72 |
| 32.06 | 64.22 | 100.31 | |
| 38.43 | 71.16 | 101.74 | |
| Isoquercitrin (3) (10.42 µg/mL) | 8.32 | 18.81 | 100.84 |
| 10.42 | 20.73 | 98.94 | |
| 12.48 | 23.12 | 101.76 | |
| Quercitrin (4) (120.25 µg/mL) | 96.16 | 215.93 | 99.50 |
| 120.20 | 241.75 | 101.08 | |
| 144.24 | 265.87 | 100.96 | |
| Quercetin (5) (1.14 µg/mL) | 0.92 | 2.07 | 101.09 |
| 1.11 | 2.23 | 98.20 | |
| 1.41 | 2.53 | 98.58 |
| Standards | Calibration Curve | Correlation Coefficient (R2) | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Rutin | y = 0.000190444x − 0.477933 | 0.9999 | 5–200 | 1.41 | 5.26 |
| Hyperin | y = 0.0000918087x − 1.65027 | 0.9999 | 16–200 | 1.42 | 7.68 |
| Isoquercitrin | y = 0.000180095x + 0.0143035 | 0.9999 | 1–100 | 0.35 | 1.03 |
| Quercitrin | y = 0.000177146x − 2.8326 | 0.9999 | 48–1000 | 9.13 | 33.44 |
| Quercetin | y = 0.0000435515x + 0.0374795 | 0.9999 | 0.2–8 | 0.09 | 0.22 |
| Sample | Rutin | Hyperin | Isoquercitrin | Quercitrin | Quercetin |
|---|---|---|---|---|---|
| HC01 | 0.8866 ± 0.0059 | 1.1959 ± 0.0006 | 0.5344 ± 0.0008 | 6.8148 ± 0.0322 | 0.0249 ± 0.0003 |
| HC02 | 1.5230 ± 0.0104 | 2.1001 ± 0.0057 | 0.8719 ± 0.0098 | 8.6587 ± 0.0266 | 0.0505 ± 0.0010 |
| HC03 | 1.6269 ± 0.0061 | 1.7714 ± 0.0010 | 0.5941 ± 0.0043 | 9.4315 ± 0.0011 | 0.0373 ± 0.0002 |
| HC04 | 0.4965 ± 0.0001 | 0.7087 ± 0.0009 | 0.3938 ± 0.0005 | 6.0251 ± 0.0066 | 0.0655 ± 0.0010 |
| HC05 | 0.0410 ± 0.0013 | 0.021 ± 0.0004 | 0.0265 ± 0.0002 | 0.2572 ± 0.0055 | 0.0098 ± 0.0001 |
| HC06 | 0.7678 ± 0.0016 | 1.1615 ± 0.0005 | 0.5380 ± 0.0024 | 5.3637 ± 0.0256 | 0.0234 ± 0.0002 |
| HC07 | 0.5144 ± 0.0012 | 0.6056 ± 0.0004 | 0.4051 ± 0.0017 | 4.3838 ± 0.0078 | 0.0191 ± 0.0003 |
| HC08 | 0.2340 ± 0.0001 | 0.2165 ± 0.0001 | 0.0742 ± 0.0004 | 0.4037 ± 0.0004 | 0.0165 ± 0.0001 |
| HC09 | 0.7595 ± 0.0002 | 1.6164 ± 0.0001 | 0.6194 ± 0.0002 | 7.1828 ± 0.0047 | 0.1126 ± 0.0001 |
| HC10 | 1.3060 ± 0.0080 | 2.2296 ± 0.0085 | 0.9085 ± 0.0031 | 10.8441 ± 0.0763 | 0.0336 ± 0.0000 |
| HC11 | 0.3922 ± 0.0072 | 0.5278 ± 0.0032 | 0.2566 ± 0.0023 | 4.1260 ± 0.0244 | 0.0102 ± 0.0002 |
| HC12 | 1.2201 ± 0.0035 | 1.3328 ± 0.0003 | 0.6561 ± 0.0027 | 8.8097 ± 0.0237 | 0.0299 ± 0.0003 |
| HC13 | 0.4605 ± 0.0042 | 0.4094 ± 0.0015 | 0.1632 ± 0.0011 | 3.7457 ± 0.0342 | 0.0191 ± 0.0005 |
| HC14 | 1.6428 ± 0.0048 | 2.0960 ± 0.0086 | 0.8210 ± 0.0053 | 13.1371 ± 0.0314 | 0.0450 ± 0.0004 |
| HC15 | 0.9696 ± 0.0062 | 0.8089 ± 0.0046 | 0.3204 ± 0.0032 | 5.7219 ± 0.0258 | 0.0208 ± 0.0004 |
| HC16 | 1.4710 ± 0.0236 | 1.8131 ± 0.0154 | 0.6079 ± 0.0059 | 9.4754 ± 0.1420 | 0.0298 ± 0.0004 |
| HC17 | 0.7509 ± 0.0014 | 1.0327 ± 0.0013 | 0.5109 ± 0.0029 | 4.9715 ± 0.0129 | 0.0208 ± 0.0003 |
| HC18 | 1.3927 ± 0.0091 | 1.3874 ± 0.0043 | 0.8232 ± 0.0129 | 6.8171 ± 0.0277 | 0.0167 ± 0.0010 |
| HC19 | 1.0374 ± 0.0036 | 1.7311 ± 0.0119 | 0.8956 ± 0.0095 | 6.9409 ± 0.0330 | 0.0409 ± 0.0004 |
| HC20 | 0.7412 ± 0.0208 | 1.6961 ± 0.0009 | 0.7570 ± 0.0027 | 4.3134 ± 0.0874 | 0.1265 ± 0.0012 |
| HC21 | 0.8791 ± 0.0073 | 1.6441 ± 0.0015 | 0.6607 ± 0.0050 | 7.6156 ± 0.0397 | 0.0317 ± 0.0033 |
| HC22 | 0.7867 ± 0.0123 | 1.1246 ± 0.0141 | 0.5793 ± 0.0138 | 5.9696 ± 0.0698 | 0.0255 ± 0.0012 |
| HC23 | 1.2055 ± 0.0043 | 1.6909 ± 0.0001 | 0.8860 ± 0.0030 | 8.4155 ± 0.0126 | 0.0283 ± 0.0003 |
| HC24 | 1.0392 ± 0.0028 | 1.7227 ± 0.0049 | 0.8921 ± 0.005 | 6.9002 ± 0.0233 | 0.0420 ± 0.0007 |
| HC25 | 0.5392 ± 0.0079 | 0.4673 ± 0.0044 | 0.2838 ± 0.0049 | 4.3088 ± 0.0739 | 0.0181 ± 0.0003 |
| HC26 | 0.1838 ± 0.0028 | 0.2007 ± 0.0037 | 0.0966 ± 0.0013 | 1.3089 ± 0.0109 | 0.0061 ± 0.0003 |
| HC27 | 0.7167 ± 0.0093 | 0.5610 ± 0.0095 | 0.2436 ± 0.0030 | 4.8100 ± 0.0354 | 0.0313 ± 0.0002 |
| HC28 | 0.7465 ± 0.0099 | 0.5954 ± 0.0031 | 0.2577 ± 0.0016 | 5.0576 ± 0.0707 | 0.0365 ± 0.0004 |
| HC29 | 1.5310 ± 0.0230 | 0.8166 ± 0.0065 | 0.9112 ± 0.0097 | 4.7169 ± 0.4037 | 0.0242 ± 0.0003 |
| HC30 | 1.0867 ± 0.0153 | 1.4220 ± 0.0136 | 0.8519 ± 0.0167 | 7.5632 ± 0.0410 | 0.0298 ± 0.0002 |
| HC31 | 1.5716 ± 0.0159 | 2.1276 ± 0.0065 | 0.9838 ± 0.0156 | 7.1727 ± 0.0255 | 0.0291 ± 0.0001 |
| HC32 | 1.1626 ± 0.0102 | 1.5755 ± 0.0115 | 0.6561 ± 0.0114 | 9.0582 ± 0.1171 | 0.0257 ± 0.0004 |
| P01 | 1.5938 ± 0.0032 | 0.8370 ± 0.0053 | 0.9447 ± 0.0434 | 4.5257 ± 0.0672 | 0.0248 ± 0.0001 |
| P02 | 1.0091 ± 0.0121 | 1.3975 ± 0.0111 | 0.8203 ± 0.0391 | 7.4174 ± 0.0144 | 0.0282 ± 0.0000 |
| P03 | 1.5291 ± 0.0057 | 2.1070 ± 0.0061 | 0.9120 ± 0.0070 | 7.2609 ± 0.0039 | 0.0289 ± 0.0002 |
| P04 | 1.0682 ± 0.0116 | 1.3654 ± 0.0075 | 0.5134 ± 0.0058 | 5.4733 ± 0.0364 | 0.0209 ± 0.0001 |
| Principal Components | Eigenvalue | Percentage of Variance | Cumulative Variance |
|---|---|---|---|
| 1 | 3.47 | 69.41% | 69.41% |
| 2 | 0.96 | 19.14% | 88.56% |
| 3 | 0.34 | 6.74% | 95.30% |
| 4 | 0.15 | 3.10% | 98.39% |
| 5 | 0.08 | 1.61% | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.H.; Ha, D.L.; Do, B.M.; Chau, N.T.N.; Tran, T.H.; Le, N.T.H.; Le, M.T. RP-HPLC-Based Flavonoid Profiling Accompanied with Multivariate Analysis: An Efficient Approach for Quality Assessment of Houttuynia cordata Thunb Leaves and Their Commercial Products. Molecules 2023, 28, 6378. https://doi.org/10.3390/molecules28176378
Nguyen MH, Ha DL, Do BM, Chau NTN, Tran TH, Le NTH, Le MT. RP-HPLC-Based Flavonoid Profiling Accompanied with Multivariate Analysis: An Efficient Approach for Quality Assessment of Houttuynia cordata Thunb Leaves and Their Commercial Products. Molecules. 2023; 28(17):6378. https://doi.org/10.3390/molecules28176378
Chicago/Turabian StyleNguyen, Minh Hien, Dieu Ly Ha, Binh Minh Do, Ngoc Trong Nghia Chau, Thi Huong Tran, Nguyen Thien Han Le, and Minh Tri Le. 2023. "RP-HPLC-Based Flavonoid Profiling Accompanied with Multivariate Analysis: An Efficient Approach for Quality Assessment of Houttuynia cordata Thunb Leaves and Their Commercial Products" Molecules 28, no. 17: 6378. https://doi.org/10.3390/molecules28176378