An Anionic Porous Indium-Organic Framework with Nitrogen-Rich Linker for Efficient and Selective Removal of Trace Cationic Dyes
Abstract
:1. Introduction
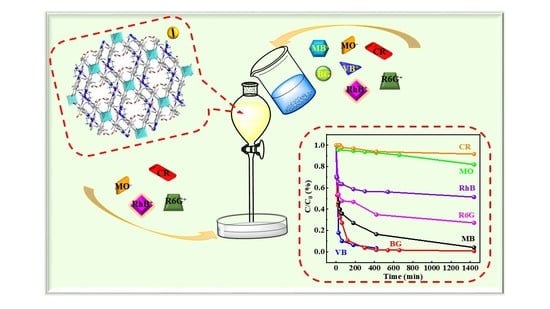
2. Results and Discussion
3. Experimental
3.1. Materials and Methods
3.2. Syntheses of HDU-1
3.3. X-ray-Crystallography
3.4. Dye Adsorption Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, E.; Kim, J.; Nam, J.; Yang, C.-M.; Jeong, H.; Kim, S.; Kang, E.; Cho, H.-J.; Choe, W. Adsorptive removal of industrial dye by nanoporous Zr porphyrinic metal-organic framework microcubes. Acs Appl. Nano Mater. 2021, 4, 10068–10076. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Sun, Z.; Han, Y.; Xu, J.; Xu, Y.; Wu, J.; Meng, H.; Zhang, X. Selective adsorption of malachite green (MG) and fuchsin acid (FA) by ZIF-67 hybridized polyvinylidene fluoride (PVDF) membranes. Dalton Trans. 2021, 5, 8927–8937. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, X.; Sun, G.; Zhao, H.; Yang, Z.; Yan, L.; Jiang, X.; Cui, Y. Highly efficient and rapid Pb(ii) removal from acidic wastewater using superhydrophilic polystyrene phosphate resin. New J. Chem. 2022, 46, 16567–16575. [Google Scholar] [CrossRef]
- Feng, L.; Zeng, T.; Hou, H. Post-functionalized metal-organic framework for effective and selective removal of Hg(II) in aqueous media. Micropor. Mesopor. Mater. 2021, 328, 111479. [Google Scholar] [CrossRef]
- Corda, N.; Kini, M.S. Recent studies in adsorption of Pb(II), Zn(II) and Co(II) using conventional and modified materials:a review. Sep. Sci. Technol. 2020, 55, 2679–2698. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M. Application of metal organic framework in wastewater treatment and detection of pollutants: Review. J. Indian. Chem. Soc. 2020, 97, 507–512. [Google Scholar]
- Wang, G.-Q.; Huang, J.-F.; Huang, X.-F.; Deng, S.-Q.; Zheng, S.-R.; Cai, S.-L.; Fan, J.; Zhang, W.G. A hydrolytically stable cage-based metal-organic framework containing two types of building blocks for the adsorption of iodine and dyes. Inorg. Chem. Front. 2021, 8, 1083–1092. [Google Scholar] [CrossRef]
- Meshram, A.A.; Sontakke, S.M. Synthesis of highly stable nanoscale MIL-53 MOF and its application for the treatment of complex mixed dye solutions and real-time dye industry effluent. Sep. Purif. Technol. 2021, 274, 119033. [Google Scholar] [CrossRef]
- Mahdavi, H.; Karami, M.; Heidari, A.A.; Kahriz, P.K. Preparation of mixed matrix membranes made up of polysulfone and MIL-53(Al) nanoparticles as promising membranes for separation of aqueous dye solutions. Sep. Purif. Technol. 2021, 274, 119073. [Google Scholar] [CrossRef]
- Li, Q.; Xue, D.; Zhang, Y.; Zhang, Z.; Gao, Z.; Bai, J. A Dual-Functional Indium−Organic Framework Towards Organic Pollutant Decontamination via Physically Selective Adsorption and Chemical Photodegradation. J. Mater. Chem. A. 2017, 5, 14182–14189. [Google Scholar] [CrossRef]
- Deng, S.; Mo, X.; Zheng, S.; Jin, X.; Gao, Y.; Cai, S.; Fan, J.; Zhang, W. Hydrolytically stable nanotubular cationic metal-organic framework for rapid and efficient removal of toxic oxo-anions and dyes from water. Inorg. Chem. 2019, 58, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sun, Q.; Liu, T.; Zhang, H.; Wang, Y.; Zhang, T.; Liu, X.; Li, W.; Zhao, Z. Highly selective adsorption of cationic dye by an anionic zinc-organic framework. Chemistryselect 2022, 7, e202202084. [Google Scholar] [CrossRef]
- Liu, H.; Gao, G.; Liu, J.; Bao, F.; Wei, Y.; Wang, H. Amide-functionalized ionic indium–organic frameworks for efficient separation of organic dyes based on diverse adsorption interactions. CrystEngComm 2019, 21, 5527–5533. [Google Scholar] [CrossRef]
- Huang, P.; Chen, C.; Wu, M.; Jiang, F.; Hong, M. An indium–organic framework for the efficient storage of light hydrocarbons and selective removal of organic dyes. Dalton T 2019, 48, 5527–5533. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, A.; Chen, W.; Shen, D.; Zhang, D.; Tong, Z.; Lee, C. Multifunctional anionic indium–organic frameworks for organic dye separation, white-light emission and dual-emitting Fe3+ sensing. J. Mater. Chem. C 2019, 7, 14897–14903. [Google Scholar] [CrossRef]
- He, Y.-C.; Yang, J.; Kan, W.-Q.; Zhang, H.-M.; Liu, Y.-Y.; Ma, J.-F. A new microporous anionic metal–organic framework as a platform for highly selective adsorption and separation of organic dyes. J. Mater. Chem. A 2015, 3, 1675–1681. [Google Scholar] [CrossRef]
- Yu, C.; Chen, J.; Zhang, Y.; Song, W.; Li, X.; Chen, F.; Zhang, Y.; Liu, D.; Liu, L. Highly efficient and selective removal of anionic dyes from aqueous solution by using a protonated metal-organic framework. J. Alloys Compd. 2021, 853, 157383. [Google Scholar] [CrossRef]
- Wan, S.; Li, L.; Liu, J.; Liu, B.; Li, G.; Zhang, L.; Liu, Y. An Imide-Decorated Indium-Organic Framework for Efficient and Selective Capture of Carcinogenic Dyes with Diverse Adsorption Interactions. Cryst. Growth Des. 2020, 20, 3199–3207. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.; Zhao, H.; Yin, S.; Wang, S.; Zhang, J.; Jiang, J.; Pan, M.; Su, C. Record high cationic dye separation performance for water sanitation using a neutral coordination framework. R J. Mater. Chem. A 2019, 7, 4751–4758. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, A.; Hu, M.; Wu, J.; Zhang, D.; Lan, Y. Assembly of Two Mesoporous Anionic Metal–Organic Frameworks for Fluorescent Sensing of Metal Ions and Organic Dyes Separation. Inorg. Chem. 2021, 60, 167–174. [Google Scholar] [CrossRef]
- Zarekarizi, F.; Morsali, A. Ultrasonic-assisted synthesis of nano-sized metal-organic framework; a simple method to explore selective and fast Congo Red adsorption. Ultrason. Sonochem. 2020, 69, 105246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Fan, N.-N.; Han, M.-L.; Yang, G.-P.; Ma, L.-F. Porous Zn(II)-Based Metal-Organic Frameworks Decorated With Carboxylate Groups Exhibiting High Gas Adsorption and Separation of Organic Dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, L.; Wu, M.K.; Han, L. A Bifunctional Anionic Metal−Organic Framework: Reversible Photochromism and Selective Adsorption of Methylene Blue. Cryst. Growth Des. 2018, 18, 5738–5744. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Masoomi, M.Y.; Morsali, A. Host−Guest Interaction Optimization through Cavity Functionalization for Ultra-Fast and Efficient Water Purification by a Metal−Organic Framework. Inorg. Chem. 2018, 57, 11578–11587. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.-N.; Han, Z.-B.; Gao, M.-L.; Cao, X.-M.; Wang, S.-M. An InIII-based Anionic Metal–Organic Framework: Sensitization of Lanthanide (III) Ions and Selective Absorption and Separation of Cationic Dyes. J. Mater. Chem. A 2015, 3, 14157–14164. [Google Scholar] [CrossRef]
- Xue, H.; Huang, X.S.; Yin, Q.; Hu, X.J.; Zheng, H.Q.; Huang, G.; Liu, T.F. Bimetallic Cationic Metal−Organic Frameworks for Selective Dye Adsorption and Effective Cr2O72− Removal. Cryst. Growth Des. 2020, 20, 4861–4866. [Google Scholar] [CrossRef]
- Deng, S.-Q.; Miao, Y.-L.; Tan, Y.-L.; Fang, H.-N.; Li, Y.-T.; Mo, X.-J.; Cai, S.-L.; Fan, J.; Zhang, W.-G.; Zheng, S.-R. An Anionic Nanotubular Metal−Organic Framework for High-Capacity Dye Adsorption and Dye Degradation in Darkness. Inorg. Chem. 2019, 58, 13979–13987. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Di, Z.; Lin, P.; Huang, P.; Wu, M.; Jiang, F.; Hong, M. An Anionic Uranium-Based Metal−Organic Framework with Ultralarge Nanocages for Selective Dye Adsorption. Cryst. Growth Des. 2018, 18, 576–580. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Eubank, J.F.; Liu, Y. Highly effective and fast removal of anionic carcinogenic dyes via an In3-cluster-based cationic metal–organic framework with nitrogen-rich ligand. Mater. Chem. Front. 2020, 4, 182–188. [Google Scholar] [CrossRef]
- Reinares-Fisac, D.; Aguirre-Diaz, L.M.; Iglesias, M.; Snejko, N.; Gutierrez-Puebla, E.; Monge, M.A.; Gandara, F. A Mesoporous Indium Metal-Organic Framework: Remarkable Advances in Catalytic Activity for Strecker Reaction of Ketones. J. Am. Chem. Soc. 2016, 138, 9089–9092. [Google Scholar] [CrossRef]
- Liu, Y.; Eubank, J.F.; Cairns, A.J.; Eckert, J.; Kravtsov, V.; Luebke, R.; Eddaoudi, M. Assembly of Metal-Organic Frameworks (MOFs) Based on Indium-Trimer Building Blocks: A Porous MOF with soc Topology and High Hydrogen Storage. Angew. Chem. Int. Ed. 2007, 46, 3278–3283. [Google Scholar] [CrossRef]
- Zou, L.; Sun, X.; Yuan, J.; Li, G.; Liu, Y. Assembly of Zeolitelike Metal-Organic Framework: An Indium-ZMOF Possessing GIS Topology and High CO2 Capture. Inorg. Chem. 2018, 57, 10679–10684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, S.; Bu, X. Multiple Functions of Ionic Liquids in the Synthesis of Three-Dimensional Low-Connectivity Homochiral and Achiral Frameworks. Angew. Chem. Int. Ed. 2008, 47, 5434–5437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Jiang, F.; Yuan, D.; Wu, M.; Zhang, S.; Zhang, L.; Hong, M. Highly Selective Carbon Dioxide Adsorption in a Water- Stable Indium-Organic Framework Material. Chem. Commun. 2012, 48, 9696–9698. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Lv, R.; Li, S.; Tang, J.; Ge, J.; Zhao, D. Two –COOH Decorated Anionic Metal-organic Frameworks with Open Cu2+ Sites Afforded Highly C2H2/CO2 and C2H2/CH4 Separation and Removal of Organic Dyes. Z. Anorg. Allg. Chem. 2019, 645, 955–959. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Ma, S.; Kong, Z.; Duan, X. Rapid adsorption and selective capture of methylene blue by anionic In-based MOF with carboxyl-decorated pore surface. Z. Anorg. Allg. Chem. 2022, 648, e202100329. [Google Scholar] [CrossRef]
- Duan, X.; Ouyang, Y.; Zeng, Q.; Ma, S.; Kong, Z.; Chen, A.; He, Z.; Yang, T.; Zhang, Q. Two carboxyl-decorated anionic metal-organic framework as solid-state electrolyte exhibiting high Li+ and Zn2+ conductivity. Inorg. Chem. 2021, 60, 11032–11037. [Google Scholar] [CrossRef]
- Duan, X.; Lv, R.; Kong, Z. An Anionic Metal-organic Framework for Selective Adsorption Separation toward Methylene Blue and Rhodamine B. Z. Anorg. Allg. Chem. 2020, 646, 1408–1411. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Cai, J.; Song, R.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A porous metal-organic framework with -COOH groups for highly efficient pollutant removal. Chem. Commun. 2014, 50, 14455–14458. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Z.; Xu, J.; Xu, W.; Sang, P.; Zhao, L.; Zhu, H.; Sun, D.; Guo, W. A NbO-type copper metal-organic framework decorated with carboxylate groups exhibiting highly selective CO2 adsorption and separation of organic dyes. J. Mater. Chem. A 2016, 4, 13844–13851. [Google Scholar] [CrossRef]









| HDU-1 | |
|---|---|
| Chemical formula | C108H92In2O28N28 |
| Formula weight | 2459.73 |
| Temperature (K) | 273(2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | C 2/c |
| a (Å) | 16.8151(12) |
| b (Å) | 19.9273(14) |
| c (Å) | 20.3388(14) |
| V (Å3) | 6642.5(8) |
| α | 90 |
| β | 102 |
| γ | 90 |
| Z | 4 |
| Density (calculated g/cm3) | 1.230 |
| Absorbance coefficient (mm−1) | 0.422 |
| F(000) | 2516 |
| Crystal size (mm3) | 0.3 × 0.21 × 0.12 |
| Goodness of fit on F2 | 1.099 |
| R1, wR2 [I > 2σ(I)] | 0.0762, 0.1802 |
| R1, wR2 (all data) | 0.0790, 0.1814 |
| Largest difference peak and hole(e/Å3) | 1.097, −2.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhang, X.; Jin, Z.; Chen, J.; Duan, X.; Ma, S.; Xia, T. An Anionic Porous Indium-Organic Framework with Nitrogen-Rich Linker for Efficient and Selective Removal of Trace Cationic Dyes. Molecules 2023, 28, 4980. https://doi.org/10.3390/molecules28134980
Feng L, Zhang X, Jin Z, Chen J, Duan X, Ma S, Xia T. An Anionic Porous Indium-Organic Framework with Nitrogen-Rich Linker for Efficient and Selective Removal of Trace Cationic Dyes. Molecules. 2023; 28(13):4980. https://doi.org/10.3390/molecules28134980
Chicago/Turabian StyleFeng, Lihui, Xiaofei Zhang, Zhekuang Jin, Jiashang Chen, Xing Duan, Shiyu Ma, and Tifeng Xia. 2023. "An Anionic Porous Indium-Organic Framework with Nitrogen-Rich Linker for Efficient and Selective Removal of Trace Cationic Dyes" Molecules 28, no. 13: 4980. https://doi.org/10.3390/molecules28134980