Two New Aluminoborates with 3D Porous-Layered Frameworks
Abstract
:1. Introduction
2. Results and Discussion
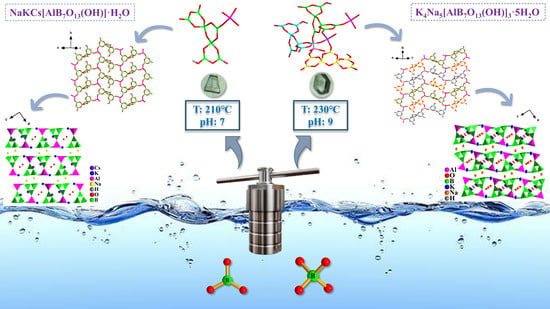
2.1. Synthesis Procedure
2.2. Structure of 1
2.3. Structure of 2
2.4. Structure Comparison
2.5. Powder XRD Patterns
2.6. IR Spectra
2.7. UV-Vis Absorption Spectra
2.8. Thermal Analysis
3. Materials and Methods
3.1. General Procedure
3.2. Syntheses
3.2.1. Syntheses of 1
3.2.2. Syntheses of 2
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lin, Z.E.; Yang, G.Y. Oxo Boron Clusters and Their Open Frameworks. Eur. J. Inorg. Chem. 2011, 2011, 3857–3867. [Google Scholar] [CrossRef]
- Wang, S.; Alekseev, E.V.; Depmeier, W.; Albrecht-Schmitt, T.E. Recent progress in actinide borate chemistry. Chem. Commun. 2011, 47, 10874–10885. [Google Scholar]
- Li, J.H.; Jiang, X.F.; Wei, Q.; Xue, Z.Z.; Wang, G.M.; Yang, G.Y. Dual-Ligand-Oriented Design of Noncentrosymmetric Complexes with Nonlinear-Optical Activity. Inorg. Chem. 2022, 61, 16509–16514. [Google Scholar] [CrossRef]
- Qiu, Q.M.; Yang, G.Y. From [B6O13]8− to [GaB5O13]8− to [Ga{B5O9(OH)}{BO(OH)2}]2−: Synthesis, structure and nonlinear optical properties of new metal borates. CrystEngComm 2021, 23, 5200–5207. [Google Scholar] [CrossRef]
- Li, X.Y.; Wei, Q.; Hu, C.L.; Pan, J.; Li, B.X.; Xue, Z.Z.; Li, X.Y.; Li, J.H.; Mao, J.G.; Wang, G.M. Achieving Large Second Harmonic Generation Effects via Optimal Planar Alignment of Triangular Units. Adv. Mater. 2022, 33, 2210718. [Google Scholar] [CrossRef]
- Dewey, C.F.; Cook, W.R.; Hodgson, R.T.; Wynne, J.J. Frequency doubling in KB5O8⋅4H2O and NH4B5O8⋅4H2O to 217.3 nm. Appl. Phys. Lett. 1975, 26, 714–716. [Google Scholar] [CrossRef]
- Wu, H.P.; Pan, S.L.; Poeppelmeier, K.R.; Li, H.Y.; Jia, D.Z.; Chen, Z.H.; Fan, X.Y.; Yang, Y.; Rondinelli, J.M.; Luo, H.S. K3B6O10Cl: A new structure analogous to perovskite with a large second harmonic generation response and deep UV absorption edge. J. Am. Chem. Soc. 2011, 133, 7786–7790. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Yang, G.Y. Syntheses, structures and optical properties of two B3O7 cluster-based borates. CrystEngComm 2022, 24, 1203–1210. [Google Scholar] [CrossRef]
- Awarded, A.H.; Honored, C.B.; Gellman, S.H. Organic Materials/ Asymmetric Catalysis/ Peptide Chemistry. Angew. Chem. Int. Ed. 2007, 46, 21. [Google Scholar]
- Pan, C.Y.; Liu, G.Z.; Zheng, S.T.; Yang, G.Y. GeB4O9·H2en: An organically templated borogermanate with large 12-ring channels built by B4O9 polyanions and GeO4 units: Host-guest symmetry and charge matching in triangular-tetrahedral frameworks. Chem. Eur. J. 2008, 14, 5057–5063. [Google Scholar]
- Wang, K.; Li, X.F.; He, C.; Li, J.H.; An, X.T.; Wei, L.; Wei, Q.; Wang, G.M. NaSb3O2(SO4)3·H2O: A New Alkali-Metal Antimony(III) Sulfate with a Unique Sb6O20H4 Unit and Moderate Birefringence. Cryst. Growth Des. 2021, 22, 478–484. [Google Scholar] [CrossRef]
- Qiu, Q.M.; Yang, G.Y. Three mixed-alkaline-metal borates with {Li@B12Ox(OH)24−x} (x = 18, 22) clusters: From isolated oxoboron cluster to unusual layer. CrystEngComm 2021, 23, 6518–6525. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, G.M. A New-Type Ultraviolet SHG Crystal-Beta-BaB2O4. Sci. Sin. Ser. B. 1985, 28, 235–243. [Google Scholar]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New nonlinear-optical crystal: LiB3O5. J. Opt. Soc. Am. B. 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Mori, I.K.Y.; Nakajima, S.; Sasaki, T.; Nakai, S. New nonlinear optical crystal: Cesium lithium borate. Appl. Phys. Lett. 1995, 67, 1818–1820. [Google Scholar] [CrossRef]
- Zhang, T.J.; Pan, R.; He, H.; Yang, B.F.; Yang, G.Y. Solvothermal Synthesis and Structure of Two New Borates Containing [B7O9(OH)5]2− and [B12O18(OH)6]6− Clusters. J. Cluster Sci. 2015, 27, 625–633. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.J.; Chen, C.A.; Yang, G.Y. Two New Borates Built by Different Types of {B9} Cluster Units. Chem. Res. Chin. Univ. 2022, 38, 744–749. [Google Scholar] [CrossRef]
- Wu, H.Q.; He, H.; Yang, B.F.; Yang, G.Y. A new mixed metal borate of BaPb[B5O9(OH)]·H2O with acentric structure. Inorg. Chem. Commun. 2013, 37, 77–79. [Google Scholar] [CrossRef]
- Liu, W.F.; Su, Z.M.; Jia, Z.Y.; Yang, G.Y. Syntheses, Structures and Characterizations of Two New Polyborates Containing Heptaborate Sub-clusters. J. Cluster Sci. 2019, 30, 1139–1144. [Google Scholar]
- Irina, V.K.; Varvara, V.A.; Grigorii, A.B.; Evgeniy, A.S.; Yulia, V.I.; Sergey, P.G. A New Approach for the Synthesis of Powder Zinc Oxide and Zinc Borates with Desired Properties. Inorganics 2022, 10, 212. [Google Scholar]
- Chen, C.A.; Liu, W.F.; Yang, G.Y. Two layered galloborates from centric to acentric structures: Syntheses and NLO properties. Chem. Commun. 2022, 58, 8718–8721. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, R.; Cheng, J.W.; He, H.; Yang, B.F.; Zhang, Q.; Yang, G.Y. A Series of Aluminoborates Templated or Supported by Zinc-Amine Complexes. Chem. Eur. J. 2015, 21, 15732–15739. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, T.J.; Ma, C.B.; Yang, G.Y. Two novel 3D borates: Porous-layer and layer-pillar frameworks. Dalton Trans. 2020, 49, 3824–3829. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, S.J.; Zhang, J.; Yang, G.Y. Extending Unique 1D Borate Chains to 3D Frameworks by Introducing Metallic Nodes. Chem. Eur. J. 2017, 23, 7614–7620. [Google Scholar] [PubMed]
- Lehmann, H.A.; Teske, K. Uber einige neue Borate des Aluminiums. Z. Anorg. Allg. Chem. 1973, 400, 169–175. [Google Scholar] [CrossRef]
- Ju, J.; Lin, J.; Li, G.; Yang, T.; Li, H.; Liao, F.; Loong, C.K.; You, L. Aluminoborate-based molecular sieves with 18-octahedral-atom tunnels. Angew. Chem. Int. Ed. 2003, 42, 5607–5610. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Yu, Z.W.; Wang, Q.; Zheng, S.T.; Pan, C.Y.; Deng, F.; Yang, G.Y. Aluminoborates with Open Frameworks: Syntheses, Structures, and Properties. Inorg. Chem. 2009, 48, 3650–3659. [Google Scholar] [CrossRef]
- Wei, L.; Wei, Q.; Lin, Z.E.; Meng, Q.; He, H.; Yang, B.F.; Yang, G.Y. A 3D aluminoborate open framework interpenetrated by 2D zinc-amine coordination-polymer networks in its 11-ring channels. Angew. Chem. Int. Ed. 2014, 53, 7188–7191. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Chen, C.A.; Chen, J.; Cheng, J.W.; Li, J.H.; Yang, G.Y. Two porous-layered borates built by B7O13(OH) clusters and AlO4/GaO4 tetrahedra. CrystEngComm 2022, 24, 8027–8033. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, J.H.; Cheng, J.W.; Yang, G.Y. Two Acentric Aluminoborates Incorporated d(10) Cations: Syntheses, Structures, and Nonlinear Optical Properties. Inorg. Chem. 2023, 62, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Pei, H.L.; Chen, C.A.; Yang, G.Y. Two Chiral Metal Complex Templated Aluminoborates Constructed from Three Types of Oxoboron Clusters. Inorg. Chem. 2021, 60, 6576–6584. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Z.; Cheng, L.; Shen, J.N.; Yang, G.Y. From discrete borate cluster to three-dimensional open framework. CrystEngComm 2013, 15, 4483–4488. [Google Scholar] [CrossRef]
- Chen, C.A.; Pan, R.; Yang, G.Y. Syntheses and structures of a new 2D layered borate and a novel 3D porous-layered aluminoborate. Dalton Trans. 2020, 49, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Pan, R.; Zhang, T.J.; Li, X.Y.; Yang, G.Y. Three Inorganic-Organic Hybrid Gallo-/Alumino-Borates with Porous-Layered Structures Containing [MB4O10(OH)] (M = Al/Ga) Cluster Units. Inorg. Chem. 2020, 59, 18366–18373. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, Q.; Wu, H.Q.; Zhou, L.J.; Yang, G.Y. Ba3M2[B3O6(OH)]2[B4O7(OH)2] (M = Al, Ga): Two novel UV nonlinear optical metal borates containing two types of oxoboron clusters. Chem. Eur. J. 2013, 19, 17662–17667. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]





| 1 | 2 | |
|---|---|---|
| Formula | NaKCsAlB7O15H3 | K4Na5Al3B21O47H13 |
| Molecular weight | 540.66 | 1346.41 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n |
| a/Å | 11.3647 (16) | 11.6261 (3) |
| b/Å | 6.9730 (8) | 20.9721 (6) |
| c/Å | 17.6729 (19) | 16.7820 (5) |
| α/° | 90 | 90 |
| β/° | 91.880 (10) | 93.646 (2) |
| γ/° | 90 | 90 |
| V/Å3 | 1399.8 (3) | 4083.6 (2) |
| Z | 4 | 4 |
| Dc/g cm−3 | 2.556 | 2.183 |
| μ/mm−1 | 3.118 | 0.699 |
| F(000) | 1016 | 2648 |
| Goodness-of-fit on F2 | 1.069 | 1.079 |
| R indices [I > 2σ(I)] 1 | 0.0484 (0.1046) | 0.0471 (0.1519) |
| R indices (all data) | 0.0751 (0.1216) | 0.0526 (0.1563) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Chen, J.; Chen, C.-A.; Wang, Z.-W.; Yang, G.-Y. Two New Aluminoborates with 3D Porous-Layered Frameworks. Molecules 2023, 28, 4387. https://doi.org/10.3390/molecules28114387
Wang C, Chen J, Chen C-A, Wang Z-W, Yang G-Y. Two New Aluminoborates with 3D Porous-Layered Frameworks. Molecules. 2023; 28(11):4387. https://doi.org/10.3390/molecules28114387
Chicago/Turabian StyleWang, Chen, Juan Chen, Chong-An Chen, Zhen-Wen Wang, and Guo-Yu Yang. 2023. "Two New Aluminoborates with 3D Porous-Layered Frameworks" Molecules 28, no. 11: 4387. https://doi.org/10.3390/molecules28114387