Comparative Virucidal Activities of Essential Oils and Alcohol-Based Solutions against Enveloped Virus Surrogates: In Vitro and In Silico Analyses
Abstract
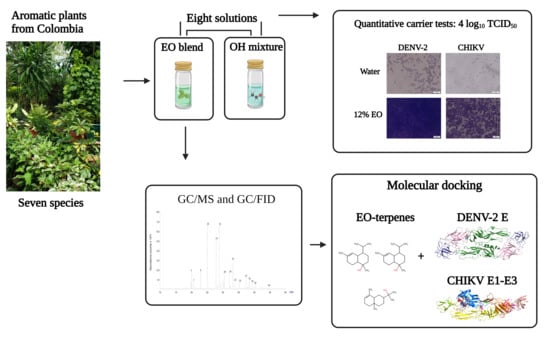
:1. Introduction
2. Results
2.1. Test Solutions
2.2. Virucidal Disinfectant Activity of the Test Solutions
2.3. Chemical Composition of the EO Blend
2.4. Molecular Interactions of EO Compounds and Viral Proteins
3. Discussion
4. Materials and Methods
4.1. Plant Material and EO Blend
4.2. Chemical Composition of the EO Blend
4.3. Preparation of the Test Solutions
4.4. Cells and Viruses
4.5. Cytotoxicity Controls
4.6. Evaluation of Virucidal Disinfectant Activity
4.7. Docking Analysis
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bhadoria, P.; Gupta, G.; Agarwal, A. Viral pandemics in the past two decades: An overview. J. Fam. Med. Prim. Care 2021, 10, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-disinfecting surfaces and infection control. Colloids Surf. B 2019, 178, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, M.; Eggers, M.; Maier, S.; Kramer, A.; Dancer, S.J.; Pittet, D. Evaluation of world health organization-recommended hand hygiene formulations. Emerg. Infect. Dis. 2020, 26, 2064–2068. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View 2020, 1, e16. [Google Scholar] [CrossRef]
- Kampf, G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018, 98, 331–338. [Google Scholar] [CrossRef]
- Dhama, K.; Patel, S.K.; Kumar, R.; Masand, R.; Rana, J.; Yatoo, M.I.; Tiwari, R.; Sharun, K.; Mohapatra, R.K.; Natesan, S.; et al. The role of disinfectants and sanitizers during COVID-19 pandemic: Advantages and deleterious effects on humans and the environment. Environ. Sci. Pollut. Res. 2021, 28, 34211–34228. [Google Scholar] [CrossRef]
- Mahmood, A.; Eqan, M.; Pervez, S.; Alghamdi, H.A.; Tabinda, A.B.; Yasar, A.; Brindhadevi, K.; Pugazhendhi, A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 2020, 742, 140561. [Google Scholar] [CrossRef]
- Jing, J.L.J.; Pei Yi, T.; Bose, R.J.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand Sanitizers: A review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. [Google Scholar] [CrossRef]
- Manion, C.R.; Widder, R.M. Essentials of essential oils. Am. J. Health Syst. Pharm. 2017, 74, 153–162. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and virucidal properties of essential oils and isolated compounds—A scientific approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari-Lajayer, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, J.S.; Visentainer, J.V.; da Silva, A.L.B.R.; Rodrigues, C. Application of essential oils as sanitizer alternatives on the postharvest washing of fresh produce. Food Chem. 2023, 407, 135101. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Bailey, E.S.; Curcic, M.; Biros, J.; Erdogmuş, H.; Bac, N.; Sacco, A. Essential oil disinfectant efficacy against SARS-CoV-2 microbial surrogates. Front. Public Health 2021, 9, 2054. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Iacovelli, F.; Scagnolari, C.; Scordio, M.; Frasca, F.; Condò, R.; Ammendola, S.; Gaziano, R.; Anselmi, M.; Divizia, M.; et al. Potential use of tea tree oil as a disinfectant agent against coronaviruses: A combined experimental and simulation study. Molecules 2022, 27, 3786. [Google Scholar] [CrossRef] [PubMed]
- Rabenau, H.F.; Schwebke, I.; Blümel, J.; Eggers, M.; Glebe, D.; Rapp, I.; Sauerbrei, A.; Steinmann, E.; Steinmann, J.; Willkommen, H.; et al. Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine: As of December 1st, 2014, prepared by the German Association for the Control of Virus Diseases (DVV) and the Robert Koch Institute (RKI). Bundesgesundheitsblatt Gesundh. 2020, 63, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. 2011, 40, 043101. [Google Scholar] [CrossRef]
- Wallace, W. NIST Standard Reference Database 1A; Version 2.3; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Nasar, S.; Rashid, N.; Iftikhar, S. Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: A review. J. Med. Virol. 2020, 92, 941–955. [Google Scholar] [CrossRef]
- Silva-Trujillo, L.; Quintero-Rueda, E.; Stashenko, E.E.; Conde-Ocazionez, S.; Rondón-Villarreal, P.; Ocazionez, R.E. Essential oils from Colombian plants: Antiviral potential against dengue virus based on chemical composition, in vitro and in silico analyses. Molecules 2022, 27, 6844. [Google Scholar] [CrossRef]
- Vaney, M.C.; Duquerroy, S.; Rey, F.A. Alphavirus structure: Activation for entry at the target cell surface. Curr. Opin. Virol. 2013, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chaurasia, P.K.; Bharati, S.L.; Golla, U.R. A mini-review on the safety profile of essential oils. MOJ Biol. Med. 2022, 7, 33–36. [Google Scholar]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent advances in the application of antibacterial complexes using essential oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Valero-Rello, A.; Sanjuán, R. Enveloped viruses show increased propensity to cross-species transmission and zoonosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2215600119. [Google Scholar] [CrossRef]
- Hitakarun, A.; Williamson, M.K.; Yimpring, N.; Sornjai, W.; Wikan, N.; Arthur, C.J.; Pompon, J.; Davidson, A.D.; Smith, D.R. Cell type variability in the incorporation of lipids in the Dengue virus virion. Viruses 2022, 14, 2566. [Google Scholar] [CrossRef]
- Sousa, I.P., Jr.; Carvalho, C.A.M.; Gomes, A.M.O. Current understanding of the role of cholesterol in the life cycle of alphaviruses. Viruses 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ge, P.; Yu, X.; Brannan, J.M.; Bi, G.; Zhang, Q.; Schein, S.; Zhou, Z.H. CryoEM structure of the mature dengue virus at 3.5-å resolution. Nat. Struct. Mol. Biol. 2013, 20, 105–110. [Google Scholar] [CrossRef]
- Rey, F.A.; Lok, S.M. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell 2018, 172, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Subbarao, N.; Rajala, M.S. Envelope proteins as antiviral drug target. J. Drug Target. 2020, 10, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Flechas, M.C.; Ocazionez, R.E.; Stashenko, E.E. Evaluation of in vitro antiviral activity of essential oil compounds against Dengue Virus. Pharmacogn. J. 2018, 10, 55–59. [Google Scholar] [CrossRef]
- Naresh, P.; Selvaraj, A.; Shyam-Sundar, P.; Murugesan, S.; Sathianarayanan, S.; Namboori, P.K.K.; Jubie, S. Targeting a conserved pocket (n-octyl-β-d–glucoside) on the dengue virus envelope protein by small bioactive molecule inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 4866–4878. [Google Scholar] [CrossRef]
- Mangala, P.V.; Blijleven, J.S.; Smit, J.M.; Lee, K.K. Visualization of conformational changes and membrane remodeling leading to genome delivery by viral class-II fusion machinery. Nat. Commun. 2022, 13, 4772. [Google Scholar] [CrossRef]
- Golin, A.P.; Choi, D.; Ghahary, A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control 2020, 48, 1062–1067. [Google Scholar] [CrossRef]
- Welch, J.L.; Xiang, J.; Okeoma, C.M.; Schlievert, P.M.; Stapleton, J.T. Glycerol monolaurate, an analog to a factor secreted by lactobacillus, is virucidal against enveloped viruses, including HIV-1. mBio 2020, 11, e00686-20. [Google Scholar] [CrossRef]
- Hernández, R.; Pájaro, N.; Caballero, K.; Stashenko, E.E.; Olivero, J. Essential Oils from plants of the genus Cymbopogon as natural insecticides to control stored product pest. J. Stored Prod. Res. 2015, 62, 81–83. [Google Scholar] [CrossRef]
- Pinzón, J.A.; Contreras, N.J.; Durán, D.C.; Martínez, J.R.; Stashenko, E.E. Green biomass production and quality of essential oils of palmarrosa (Cymbopogon martinii Roxb.) with application of synthetic fertilizers and organic fertilizers. Acta Agron. 2014, 63, 335–342. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Cala, M.; Durán, D.C.; Caballero, D. Chromatographic and mass spectrometric characterization of essential oils and extracts from Lippia (Verbenaceae) aromatic plants. J. Sep. Sci. 2013, 36, 192–202. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Medina, J.D.; Durán, D.C. Analysis of essential oils isolated by steam distillation from Swinglea glutinosa fruits and leaves. J. Essent. Oil Res. 2015, 27, 276–282. [Google Scholar] [CrossRef]
- Carreño, M.F.; Jiménez-Silva, C.L.; Rey-Caro, L.A.; Conde-Ocazionez, S.A.; Flechas-Alarcón, M.C.; Velandia, S.A.; Ocazionez, R.E. Dengue in Santander State, Colombia: Fluctuations in the prevalence of virus serotypes are linked to dengue incidence and genetic diversity of the circulating viruses. Trop. Med. Int. Health 2019, 12, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. WJV World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed]



| No. | Content | DMSO, % | Identifier |
|---|---|---|---|
| 1 | 12% EO + 1% OH | 7.9 | 12EO + 1OH |
| 2 | 12% EO + 5% OH | 7.6 | 12EO + 5OH |
| 3 | 12% EO + 10% OH | 7.2 | 12EO + 10OH |
| 4 | 6% EO + 10% OH | 7.2 | 6EO + 10OH |
| 5 | 3% EO + 10% OH | 1.8 | 3EO + 10OH |
| 6 | 12% EO | 8.0 | 12EO |
| 7 | 3% EO | 2.0 | 3EO |
| 8 | 10% OH | - | 10OH |
| Solution | Dilution/Percentage of Viability | ||
|---|---|---|---|
| 1:10 | 1:100 | 1:1000 | |
| 12EO | 80 ± 26 | 90 ± 13 | 100 ± 10 |
| 12EO + 1OH | 80 ± 11 | 92 ± 8.4 | 96 ± 6.0 |
| 12EO + 5OH | 80 ± 16 | 93 ± 7.3 | 97 ± 2.7 |
| 12EO + 10OH | 80 ± 25 | 90 ± 10 | 100 ± 10 |
| 6EO + 10OH | 92 ± 9.2 | 96 ± 7.1 | 98 ± 3.3 |
| 3EO + 10OH | 92 ± 8.9 | 95 ± 9.1 | 95 ± 7.3 |
| 3EO | 100 ± 0.0 | 94 ± 8.9 | 90 ± 33 |
| 10OH | 94 ± 5.4 | 90 ± 8.8 | 90 ± 10 |
| Acetic acid | 0.0 ± 3.2 | 80 ± 32 | 93 ± 4.9 |
| Solution | DENV-2: TCID50/mL | CHIKV: TCID50/mL | ||||
|---|---|---|---|---|---|---|
| Log10 | RF | % R | Log10 | RF | % R | |
| Water | 3.8 ± 0.4 † and 5 ± 1.2 | 5.3 ± 0.5 | - | - | ||
| Acetic acid | 0.0 | 4.9 ± 0.0 | 100 | 0.0 | 5.3 ± 0.5 | 100 |
| 12EO | 1 ± 1.6 | 3.9 ± 0.4 | 81.6 | 5.3 ± 0.5 | 0.0 | 0.0 |
| 12EO + 10OH | 0.0 | 4.9 ± 0.0 | 100 | 4.3 ± 0.7 | 0.9 ± 0.7 | 18.8 |
| 12EO + 5OH | 0.0 | 4.9 ± 0.0 | 100 | 3.1 ± 0.2 * | 2.2 ± 0.2 | 41.5 |
| 12EO + 10OH | 0.0 | 4.9 ± 0.0 | 100 | 0.0 | 5.3 ± 0.0 | 100 |
| 6EO + 10OH | 0.0 | 4.9 ± 0.0 | 100 | 5.7 ± 0.2 | 0.0 | 0.0 |
| 3EO + 10OH | 3.5 ± 0.7 | 0.5 ± 0.7 | 28.5 | 5 ± 1.0 | 0.4 ± 1.0 | 9.5 |
| 3EO | 3.1 ± 0.3 | 0.7 ± 0.6 | 18.4 | ND | ND | - |
| 10OH | 4.2 ± 0.0 | 0.7 ± 0.0 | 0.0 | 5.3 ± 0.5 | 0.0 | 0.0 |
| Solution | DENV-2: Log10 TCID50/mL | CHIKV: Log10 TCID50/mL | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Treated | RF | R, % | Control | Treated | RF | R, % | |
| 12EO | ||||||||
| 5 min | 5.4 ± 0.2 | 0.0 | 5.4 ± 0.2 | 100 | 5.7 ± 0.7 | 3.0 ± 0.7 * | 2.7 ± 0.1 | 47.3 |
| 10 min | 5.0 ± 1.0 | 0.0 | 5.0 ± 1.5 | 100 | 4.8 ± 0.4 | 0.0 | 4.8 ± 0.3 | 100 |
| 20 min | 5.4 ± 0.5 | 0.0 | 5.4 ± 0.5 | 100 | 4.9 ± 0.1 | 0.0 | 4.0 ± 0.1 | 100 |
| 30 min | 4.5 ± 0.9 | 0.0 | 4.5 ± 0.9 | 100 | 4.6 ± 0.5 | 0.0 | 4.6 ± 0.5 | 100 |
| 3EO | ||||||||
| 5 min | 4.0 ± 0.1 | 3.0 ± 0.6 | 0.9 ± 0.5 | 25 | 6.1 ± 0.6 | 5.4 ± 0.5 | 0.6 ± 0.6 | 11.4 |
| 10 min | 5.0 ± 1.5 | 3.3 ± 0.6 | 1.0 ± 0.7 | 26.6 | 5.1 ± 0.2 | 5.1 ± 0.4 | 0.0 | 0.0 |
| 20 min | 3.7 ± 0.1 | 2.1 ± 0.8 | 0.4 ± 1.0 | 43.2 | 6.1 ± 0.3 | 3.3 ± 0.7 * | 2.8 ± 0.8 | 45.9 |
| 30 min | 3.7 ± 0.4 | 0.0 ± 1.4 | 3.7 ± 0.1 | 100 | 5.0 ± 1.5 | 0.0 ± 0.0 | 5.0 ± 1.5 | 100 |
| No. | Compound | Type | Linear Retention Indices | GC/FID Relative Peak Area, % | |
|---|---|---|---|---|---|
| Exp. | Lit. | ||||
| 1 | α-Pinene * | MH | 934 | 932 a | 0.1 |
| 2 | 6-Methyl-hept-5-en-2-one | OC | 984 | 985 a | 0.1 |
| 3 | β-Myrcene * | MH | 989 | 990 a | 0.2 |
| 4 | p-Cymene * | MH | 1026 | 1027 a | 0.8 |
| 5 | Limonene * | MH | 1031 | 1029 a | 2.0 |
| 6 | 1,8-Cineole * | OM | 1036 | 1034 a | 0.3 |
| 7 | trans-β-Ocimene | MH | 1047 | 1050 a | 0.9 |
| 8 | γ-Terpinene * | MH | 1060 | 1059 a | 0.2 |
| 9 | Linalool * | OM | 1100 | 1096 a | 2.2 |
| 10 | Citronellal * | OM | 1157 | 1153 a | 22.6 |
| 11 | Isopulegol | OM | 1165 | 1155 a | 0.3 |
| 12 | n-Decanal | OC | 1207 | 1201 a | 0.1 |
| 13 | Citronellol | OM | 1220 | 1233 a | 14.1 |
| 14 | Neral | OM | 1241 | 1242 b | 0.4 |
| 15 | Geraniol * | OM | 1258 | 1255 b | 35.4 |
| 16 | Geranial * | OM | 1271 | 1270 b | 0.8 |
| 17 | Thymol * | PhC | 1292 | 1290 a | 1.1 |
| 18 | Carvacrol * | PhC | 1301 | 1298 a | 2.1 |
| 19 | Citronellyl acetate | OM | 1346 | 1350 a | 2.5 |
| 20 | Eugenol | PhC | 1354 | 1356 a | 0.4 |
| 21 | Geranyl acetate | OM | 1377 | 1379 a | 4.7 |
| 22 | β-Elemene | SH | 1396 | 1389 a | 1.0 |
| 23 | trans-β-Caryophyllene * | SH | 1432 | 1428 d | 0.9 |
| 24 | α-Guaieno | SH | 1444 | 1440 b | 0.1 |
| 25 | α-Humulene * | SH | 1468 | 1465 d | 0.2 |
| 26 | γ-Muurolene | SH | 1484 | 1478 a | 0.1 |
| 27 | Germacrene D * | SH | 1492 | 1481 a | 1.5 |
| 28 | α-Muurolene | SH | 1506 | 1500 a | 0.4 |
| 29 | α-Bulnesene | SH | 1511 | 1509 a | 0.1 |
| 30 | γ-Cadinene | SH | 1523 | 1513 a | 0.3 |
| 31 | δ-Cadinene | SH | 1526 | 1522 a | 1.1 |
| 32 | Elemol | OS | 1557 | 1548 a | 0.9 |
| 33 | trans-Nerolidol * | OS | 1565 | 1561 a | 0.2 |
| 34 | Germacrene D-4-ol | OS | 1578 | 1574 a | 0.7 |
| 35 | Caryophyllene oxide * | OS | 1586 | 1582 a | 0.1 |
| 36 | epi-α-Cadinol | OS | 1653 | 1650 c | 0.1 |
| 37 | epi-α-Muurolol | OS | 1655 | 1642 c | 0.2 |
| 38 | α-Cadinol | OS | 1667 | 1653 c | 0.2 |
| 39 | α-Eudesmol | OS | 1669 | 1659 c | 0.1 |
| 40 | Patchoulol | OS | 1691 | 1660 b | 0.1 |
| 41 | Farnesol | OS | 1719 | 1723 b | 0.1 |
| 42 | Neryl hexanoate | OM | 1750 | 1732 c | 0.2 |
| 1. Monoterpenoids | 87.7 | ||||
| 1.1 Monoterpene hydrocarbons (MH) | 4.2 | ||||
| 1.2 Oxygenated monoterpenes (OM) | 83.5 | ||||
| Alcohols | 52 | ||||
| Acetates | 7.2 | ||||
| Aldehydes | 23.8 | ||||
| Others (ethers, esters, epoxides) | 0.5 | ||||
| 2. Sesquiterpenoids | 8.4 | ||||
| 2.1 Sesquiterpene hydrocarbons (SH) | 5.7 | ||||
| 2.2 Oxygenated sesquiterpenes (OS) | 2.7 | ||||
| Alcohols | 2.6 | ||||
| Others (Oxides) | 0.1 | ||||
| 3. Phenolic compounds (PhC) (Thymol, carvacrol, eugenol) | 3.6 | ||||
| 4. Other oxygenated compounds (OC) (n-Decanal, 6-methyl-5-hepten-2-one) | 0.2 | ||||
| Compound | Structural Formula | Amino Acid Residues. H-Bond in Bold Font | Kcal/mol |
|---|---|---|---|
| Isopulegol |  | Thr189, Leu191, Phe193, Leu207, Phe279 | −6.70 ± 0.6 |
| Farnesol |  | Thr48, Leu135, Tyr137, Gly190, Leu191, Phe193, Leu207, Phe279, His282, Leu283 | −6.57 ± 0.6 |
| α-Cadinol |  | Thr48, Tyr137, Thr189, Leu191, Phe193, Leu207, Phe279 | −6.54 ± 0.5 |
| Eugenol |  | Thr48, Leu135, Thr189, Leu191, Phe193, Phe279, Gly281, His282 | −6.54 ± 0.5 |
| α-Eudesmol |  | Thr48, Val130, Phe193, Leu207, Phe279, Leu283 | −6.50 ± 0.6 |
| Compound | Structural Formula | Protein | Amino Acid Residues. H-Bond in Bold Font | Kcal/mol |
|---|---|---|---|---|
| α-Cadinol |  | E1, domain II | Asn39, Thr42, Pro133, Pro134, Lys241, Leu244 | −6.70 ± 0.2 |
| α-Eudesmol |  | E2, β-ribbon connector | Pro133, Val135, Ile136, Lys140, Phe141 | −6.70 ± 0.3 |
| Caryophyllene oxide |  | E2, β-ribbon connector | Arg104, Pro133, Val135, Ile136, Lys40, Phe141 | −6.50 ± 0.31 |
| Patchoulol |  | E2, β-ribbon connector | Pro133, Ile136, Lys140, Phe141, Asp43 | −6.45 ± 0.2 |
| α-Guaiene |  | E1, domain II | Pro134, Lys241, Tyr242, Lys245 | −6.38 ± 0.2 |
| Germacrene D-4-ol |  | E2, β-ribbon connector | Thr42, Pro134, Lys241, Leu244, Lys245, Asn39 | −6.38 ± 0.2 |
| epi-α-Muurolol |  | E1, domain II | Pro134, Lys241, Leu244, Lys245 | −6.37 ± 0.2 |
| α-Humulene |  | E2, β-ribbon connector | Ile136, Phe141 | −6.37 ± 0.3 |
| trans-β-Caryophyllene |  | E2, β-ribbon connector | Ile136, Phe141, Arg144 | −6.32 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Acevedo, V.; Ocazionez, R.E.; Stashenko, E.E.; Silva-Trujillo, L.; Rondón-Villarreal, P. Comparative Virucidal Activities of Essential Oils and Alcohol-Based Solutions against Enveloped Virus Surrogates: In Vitro and In Silico Analyses. Molecules 2023, 28, 4156. https://doi.org/10.3390/molecules28104156
Parra-Acevedo V, Ocazionez RE, Stashenko EE, Silva-Trujillo L, Rondón-Villarreal P. Comparative Virucidal Activities of Essential Oils and Alcohol-Based Solutions against Enveloped Virus Surrogates: In Vitro and In Silico Analyses. Molecules. 2023; 28(10):4156. https://doi.org/10.3390/molecules28104156
Chicago/Turabian StyleParra-Acevedo, Valentina, Raquel E. Ocazionez, Elena E. Stashenko, Lina Silva-Trujillo, and Paola Rondón-Villarreal. 2023. "Comparative Virucidal Activities of Essential Oils and Alcohol-Based Solutions against Enveloped Virus Surrogates: In Vitro and In Silico Analyses" Molecules 28, no. 10: 4156. https://doi.org/10.3390/molecules28104156