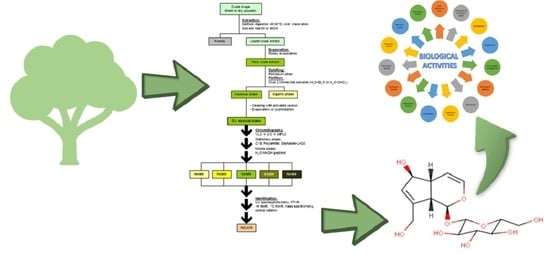
Characteristics, Isolation Methods, and Biological Properties of Aucubin
Abstract
:1. Introduction
2. Physicochemical Characteristics
3. Aucubin-Producing Plants
4. Isolation of Aucubin
5. Biological Properties
5.1. Anti-Inflammatory Activities
| No | Compound | In Vitro/In Vivo | Cell or Animal Model | Concentration/Dose | Administration Route | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aucubin | In vitro | 3T3-L1 adipocytes, stimulated using 10 ng/mL TNF-α | 1, 3, 10, 30 µM | N/A | [53] |
| 2 | Aucubin | In vitro | Murine chondrocytes, stimulated using 10 ng/mL IL-1β | 1, 10, 20, 50 µM | N/A | [54] |
| 3 | Aucubin | In vitro | THP-1 macrophages, stimulated using LPS 5 µg/mL | 10, 25, 50, 100, 300 µg/mL | N/A | [55] |
| 4 | Aucubin | In vivo | Normal C57BL/6 male mice, diabetes was induced using a high-fat diet and streptozotocin | 20, 40, 80 mg/kg BW | p.o. | [56] |
| 5 | Aucubin | In vivo | Male Kunming mice; gastric mucosal injury was induced using 70% ethanol | 20, 40, 80 mg/kg BW | i.g. | [57] |
| 6 | Aucubin | In vivo | Mouse model of epileptic ICR with pilocarpine at 320 mg/kg BW | 50, 100 mg/kg BW | i.p. | [58] |
| 7 | Aucubin | In vivo | Male BALB/c mice; induced using cisplatin | 1, 5, 5 mg/kg BW | p.o. i.p. | [61] |
| 8 | Aucubin | In vitro | Neuron cells, stimulated using H2O2 | 50, 100, 200 µg/mL | N/A | [59] |
| 9 | Aucubin | In vivo | Male and pregnant C57BL/6 mice with traumatic brain injury | 20, 40 mg/kg BW | i.p. | [59] |
| 10 | Aucubin | In vitro | 3T3-L1 cells, stimulated using apoC-III | 35, 70, 140, 280 µg/mL | N/A | [62] |
| 11 | Aucubin | In vivo | C57/BL6 mice, administered tyloxapol with/without aucubin using intraperitoneal injection | 10, 20, 40 mg/kg BW | i.p. | [62] |
| 12 | Aucubin | In vitro | Neonatal rat cardiomyocytes, stimulated using 10 µg/mL LPS | 5,15, 45 µM | N/A | [63] |
| 13 | Aucubin | In vivo | C57BL/6 mice, stimulated using LPS at 6 mg/kg BW | 20, 80 mg/kg BW | Gavage | [63] |
| 14 | Aucubin | In vitro | Human corneal cells, subjected to desiccation stress | 0,1, 1, 7, 15 µg/mL | N/A | [60] |
| 15 | Aucubin | In vivo | Male rats that had their left exorbital lacrimal gland removed (mouse model of dry eye disease) | 75 mg/kg BW | p.o. | [60] |
| 16 | Aucubin | In vitro | Human hepatocyte HL7702 (LO2), overexpression of TLR-4 | 2, 4, 8, 16, 32, 64, 128, 256 µM | N/A | [64] |
| 17 | Aucubin | In vivo | Sprague–Dawley rats with IRL condition | 1, 5, 10 mg/kg BW | i.p. | [64] |
| 18 | Aucubin | In vitro | RAW264.7 cells and macrophage-like THP-1 cells | 50, 100 µM | N/A | [65] |
| 19 | Aucubin | In vivo | Wild-type (WT) male C57BL/6 J mice and Nrf2 knockout mice, induced using LPS | 10, 20 mg/kg BW | i.p. | [65] |
5.2. Antioxidant
| No | Compound | In Vitro/In Vivo | Cell or Animal Model | Concentration/Dose | Administration Route | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aucubin and aucubigenin | In vitro | LX-2 cells (human hepatic stellate cell lines), induced using TGF-β1 | Aucubin: 1, 10, 100, 200, 400, 800 µM Aucubigenin: 100 µM | N/A | [68] |
| 2 | Aucubin | In vitro | MC3T3-E1 (murine osteoblastic cell lines), induced using Ti particles | 0.1, 1, 10 µM | N/A | [69] |
| 3 | Aucubin | In vivo | Wild-type (WT) C57BL/6 and Nrf2 knockout mice, induced using LPS | 10, 20 mg/kg BW | i.p. | [65] |
| 4 | Aucubin | In vivo | C57BL/6 mice, diabetes was induced using a high-fat diet and streptozotocin | 20, 40, 80 mg/kg BW | p.o. | [56] |
| 5 | Aucubin | In vivo | Kunming mice, gastric mucosal lesions were induced using 70% ethanol | 20, 40, 80 mg/kg BW | i.g. | [57] |
| 6 | Aucubin | In vitro | Neuron cells, stimulated using 100 µM H2O2 | 50, 100, 200 µg/mL | N/A | [59] |
| 7 | Aucubin | In vivo | C57BL/6 mice, traumatic brain injury was induced using lentivirus at 4 µL | 20, 40 mg/kg BW | i.p. | [59] |
| 8 | Aucubin | In vitro | 3T3-L1 cells, stimulated using apolipoprotein C-III at 100 µg/mL | 35, 70, 140, 280 µg/mL | N/A | [62] |
| 9 | Aucubin | In vivo | C57BL/6 mice, stimulated using tyloxapol at 300 mg/kg BW | 10, 20, 40 mg/kg BW | i.p. | [62] |
| 10 | Aucubin | In vitro | Sertoli cells (primary cells and the cell line TM4), induced using 0.5 μM triptolide | 5, 10, 20 µM | N/A | [70] |
| 11 | Aucubin | In vivo | Mice, induced using triptolide at 120 µg/kg BW | 5, 10, 20 mg/kg BW | i.p. | [70] |
| 12 | Aucubin | In vitro | H9c2 cells, exposed to hypoxia | 10, 50 µM | N/A | [71] |
| 13 | Aucubin | In vivo | C57BL/6 mice by inducing myocardial infarction | 10 mg/kg BW | i.p. | [71] |
| 14 | Aucubin | In vitro | MG63 cells (human osteoblast-like cells), stimulated using dexamethasone or H2O2 | 1, 2.5, 5 µM | N/A | [72] |
| 15 | Aucubin | In vivo | C57BL/6 mice, stimulated using dexamethasone at 30 mg/kg BW | 5, 15, 45 mg/kg BW | i.g. | [72] |
| 16 | Aucubin | In vivo | Sprague–Dawley rats with liver ischemia–reperfusion injury | 1, 5, 10 mg/kg BW | i.p. | [64] |
| 17 | Aucubin | In vitro | Neonatal rat cardiomyocytes, stimulated using LPS at 10 µg/mL | 5, 15, 45 µM | N/A | [63] |
| 18 | Aucubin | In vivo | C57BL/6 mice, stimulated using LPS at 6 mg/kg BW | 20, 80 mg/kg BW | Gavage | [63] |
| 19 | Aucubin | In vitro | Mouse chondrocytes, induced using IL-1β | 10, 20, 50 µM | N/A | [73] |
5.3. Anxiolytic and Antidepressant
5.4. Antidiabetic
5.5. Antifibrotic
5.6. Antifungal and Antibacterial
5.7. Antihyperlipidemic
5.8. Anticancer
5.9. Gastroprotective
5.10. Hepatoprotective
5.11. Cardioprotective
5.12. Neuroprotective
5.13. Osteoprotective
5.14. Renoprotective
5.15. Retinoprotective
6. Safety and Toxicity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M. Terpenoids. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–266. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). Physiol. Mol. Biol. Plants 2000, 24, 1250–1319. [Google Scholar]
- Kartini Piyaviriyakul, S.; Siripong, P.; Vallisuta, O. HPTLC simultaneous quantification of triterpene acids for quality control of Plantago major L. and evaluation of their cytotoxic and antioxidant activities. Ind. Crops Prod. 2014, 60, 239–246. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Aucubin [Online]. 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Aucubin (accessed on 16 July 2021).
- American Chemical Society. Aucubin [Online]. 2021. Available online: https://commonchemistry.cas.org/detail?cas_rn=479-98-1 (accessed on 16 July 2021).
- United States National Library of Medicine. Aucubin [Online]. 2021. Available online: https://chem.nlm.nih.gov/chemidplus/sid/0000479981 (accessed on 16 July 2021).
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Akdemir, Z.Ş.; Tatli, İ.İ.; Bedir, E.; Khan, I.A. Acylated iridoid glycosides from Verbascum lasianthum. Turk. J. Chem. 2004, 28, 101–110. [Google Scholar]
- Li, Y.; Zhao, Y.; Zhang, Y.-M.; Wang, M.-J.; Sun, W.-J. X-ray crystal structure of iridoid glucoside aucubin and its aglycone. Carbohydr. Res. 2009, 344, 2270–2273. [Google Scholar] [CrossRef]
- Suh, N.; Shim, C.; Lee, M.H.; Kim, S.K.; Chang, I. Pharmacokinetic study of an iridoid glucoside: Aucubin. Pharm. Res. 1991, 8, 1059–1063. [Google Scholar] [CrossRef]
- Park, K.S. An overview on anti-inflammatory activities of Aucubin. Int. J. Herb. Med. 2020, 8, 45–48. [Google Scholar]
- Trim, A.R.; Hill, R. The preparation and properties of aucubin, asperuloside and some related glycosides. Biochem. J. 1952, 50, 310–319. [Google Scholar] [CrossRef]
- Ho, J.N.; Lee, Y.H.; Park, J.S.; Jun, W.J.; Kim, H.K.; Hong, B.S.; Shin, D.H.; Cho, H.Y. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol. Pharm. Bull. 2005, 28, 1244–1248. [Google Scholar] [CrossRef]
- Chu, H.; Li, R.; Gao, Y.; Li, Q. Evaluation of the anxiolytic and antidepressant activities of aucubin in mice. Acta Pol. Pharm. Drug Res. 2020, 77, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, M.; Tian, H.; Liu, T.; Yang, L. Enrichment and purification of aucubin from Eucommia ulmoides ionic liquid extract using macroporous resins. Materials 2018, 11, 1758. [Google Scholar] [CrossRef] [PubMed]
- Pastene-Navarrete, E.; Torres-Vega, J. Buddleja globosa Hope. In Medicinal and Aromatic Plants of South America Vol. 2: Argentina, Chile and Uruguay; Springer: Cham, Switzerland, 2021; pp. 135–144. [Google Scholar]
- El-Domiaty, M.M.; Wink, M.; Aal, M.M.A.; Abou-Hashem, M.M.; Abd-Alla, R.H. Antihepatotoxic activity and chemical constituents of Buddleja asiatica Lour. Z. Nat. C 2009, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kuruüzüm-Uz, A.; Ströch, K.; Demirezer, L.Ö.; Zeeck, A. Glucosides from Vitex agnus-castus. Phytochemistry 2003, 63, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Damtoft, S.; Jensen, S.R.; Nielsen, B.J. Iridoid glucosides from Utricularia australis and Pinguicula vulgaris (lentibulariaceae). Phytochemistry 1985, 24, 2281–2283. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Bianco, A. Iridoids from Bellardia trixago (L.). All. Nat. Prod. Res. 2013, 27, 1413–1416. [Google Scholar] [CrossRef]
- Carrillo-Ocampo, D.; Bazaldúa-Gómez, S.; Bonilla-Barbosa, J.R.; Aburto-Amar, R.; López, V.R. Anti-inflammatory activity of iridoids and verbascoside isolated from Castilleja tenuiflora. Molecules 2013, 18, 12109–12118. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Ma, X.; Zhu, C.; Zhao, X.; Du, H.; Chen, Z.; He, S. Comparison of Catalpol and Aucubin Contents in Different Parts of Wild Centranthera grandiflora. China Pharm. 2019, 2623–2627. [Google Scholar]
- Kirmizibekmez, H.; Atay, I.; Kaiser, M.; Brun, R.; Cartagena, M.M.; Carballeira, N.M.; Yesilada, E.; Tasdemir, D. Antiprotozoal activity of Melampyrum arvense and its metabolites. Phytother. Res. 2010, 25, 142–146. [Google Scholar] [CrossRef]
- Venditti, A.; Ballero, M.; Serafini, M.; Bianco, A. Polar compounds from Parentucellia viscosa (L.) Caruel from Sardinia. Nat. Prod. Res. 2014, 29, 602–606. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, D.; Qin, M.; Li, X.E. Simultaneous determination of catalpol, aucubin, and geniposidic acid in different developmental stages of Rehmannia glutinosa leaves by high performance liquid chromatography. J. Anal. Methods Chem. 2016, 2016, 4956589. [Google Scholar] [CrossRef] [PubMed]
- Rønsted, N.; Bello, M.A.; Jensen, S.R. Aragoside and iridoid glucosides from Aragoa cundinamarcensis. Phytochemistry 2003, 64, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Rønsted, N.; Jensen, S.R. Iridoid glucosides and caffeoyl phenylethanoid glycosides from Campylanthus salsaloides and Campylanthus glaber. Biochem. Syst. Ecol. 2002, 30, 1091–1095. [Google Scholar] [CrossRef]
- Sertić, M.; Crkvenčić, M.; Mornar, A.; Pilepić, K.H.; Nigović, B.; Maleš, Ž. Analysis of aucubin and catalpol content in different plant parts of four Globularia species. J. Appl. Bot. Food Qual. 2015, 88, 30. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Akbay, P.; Sticher, O.; Çalış, I. Iridoids from Globularia dumulosa. Z. Nat. C 2003, 58, 181–186. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Çaliş, I.; Akbay, P.; Sticher, O. Iridoid and bisiridoid glycosides from Globularia cordifolia. Z. Nat. C 2003, 58, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Serafini, M.; Nicoletti, M.; Bianco, A. Terpenoids of Linaria alpina (L.) Mill. from Dolomites, Italy. Nat. Prod. Res. 2015, 29, 2041–2044. [Google Scholar] [CrossRef]
- Albach, D.C.; Gotfredsen, C.H.; Jensen, S.R. Iridoid glucosides of Paederota lutea and the relationships between Paederota and Veronica. Phytochemistry 2004, 65, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, S.; Kemp, P.D.; Pain, S.J.; Back, P.J. Bioactive compounds, aucubin and acteoside, in plantain (Plantago lanceolata L.) and their effect on in vitro rumen fermentation. Anim. Feed. Sci. Technol. 2016, 222, 158–167. [Google Scholar] [CrossRef]
- Genç, Y.; Saraçoğlu, İ.; Nagatsu, A.; Harput, Ü.Ş. Iridoid and megastigman glucosides from Plantago lagopus L. FABAD J. Pharm. Sci. 2010, 35, 29–34. [Google Scholar]
- Taskova, R.; Handjieva, N.; Evstatieva, L.; Popov, S. Iridoid glucosides from Plantago cornuti, Plantago major and Veronica cymbalaria. Phytochemistry 1999, 52, 1443–1445. [Google Scholar] [CrossRef]
- Franzyk, H.; Husum, T.L.; Jensen, S.R. A caffeoyl phenylethanoid glycoside from Plantago myosuros. Phytochemistry 1998, 47, 1161–1162. [Google Scholar] [CrossRef]
- Jensen, S.R.; Opitz, S.E.W.; Gotfredsen, C.H. A new phenylethanoid triglycoside in Veronica beccabunga L. Biochem. Syst. Ecol. 2011, 39, 193–197. [Google Scholar] [CrossRef]
- Kroll-Møller, P.; Pedersen, K.D.; Gousiadou, C.; Kokubun, T.; Albach, D.; Taskova, R.; Garnock-Jones, P.J.; Gotfredsen, C.H.; Jensen, S. Iridoid glucosides in the genus Veronica (Plantaginaceae) from New Zealand. Phytochemistry 2017, 140, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Harput, U.S.; Nagatsu, A.; Ogihara, Y.; Saracoglu, I. Iridoid glucosides from Veronica pectinata var. glandulosa. Z. Nat. C 2003, 58, 481–484. [Google Scholar] [CrossRef]
- Sesterhenn, K.; Distl, M.; Wink, M. Occurrence of iridoid glycosides in in vitro cultures and intact plants of Scrophularia nodosa L. Plant Cell Rep. 2006, 26, 365–371. [Google Scholar] [CrossRef]
- Forgacs, P.; Provost, J.; Jehanno, A. Aucubin from Sutera dissecta. J. Nat. Prod. 1986, 49, 367. [Google Scholar] [CrossRef]
- Kupeli, E.; Tatli, I.I.; Akdemir, Z.S.; Yesilada, E. Bioassay-guided isolation of anti-inflammatory and antinociceptive glycoterpenoids from the flowers of Verbascum lasianthum Boiss. ex Bentham. J. Ethnopharmacol. 2007, 110, 444–450. [Google Scholar] [CrossRef]
- Aligiannis, N.; Mitaku, S.; Tsitsa-Tsardis, E.; Harvala, C.; Tsaknis, I.; Lalas, S.; Haroutounian, S. Methanolic extract of Verbascum macrurum as a source of natural preservatives against oxidative rancidity. J. Agric. Food Chem. 2003, 51, 7308–7312. [Google Scholar] [CrossRef]
- Akdemir, Z.; Kahraman, Ç.; Tatlı, I.I.; Akkol, E.K.; Süntar, I.; Keles, H. Bioassay-guided isolation of anti-inflammatory, antinociceptive and wound healer glycosides from the flowers of Verbascum mucronatum Lam. J. Ethnopharmacol. 2011, 136, 436–443. [Google Scholar] [CrossRef]
- Mouriès, C.; Rakotondramasy, V.C.; Libot, F.; Koch, M.; Tillequin, F.; Deguin, B. Synthesis and cytotoxicity of a novel iridoid glucoside derived from aucubin. Chem. Biodivers. 2005, 2, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Rakotondramasy, V.C.; Mouriès, C.; Cachet, X.; Neghra, A.; El Mourabet, M.; Tillequin, F.; Koch, M.; Deguin, B. A novel series of cytotoxic iridoid glucosides derived from aucubin: Design, synthesis and structure–activity relationships. Eur. J. Med. Chem. 2010, 45, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-M.; Shang, P.-P.; Hou, X.-F.; Liu, J.-B.; Sun, W.-J. Preliminary study on the stability of aucubin. Chin. J. Pharm. Anal. 2003, 23, 167–169. [Google Scholar]
- Li, H.; Hu, J.; Ouyang, H.; Li, Y.; Shi, H.; Ma, C.; Zhang, Y. Extraction of aucubin from seeds of Eucommia ulmoides Oliv. using supercritical carbon dioxide. J. AOAC Int. 2009, 92, 103–110. [Google Scholar] [CrossRef]
- Ersöz, T.; Yalcin, F.N.; Taşdemir, D.; Sticher, O.; Çaliş, İ. Iridoid and Lignan Glucosides from Bellardia trixago (L.). All. Turk. J. Med. Sci. 1998, 28, 397–400. [Google Scholar]
- Martini, F.H.; Nath, J.L.; Bartholomew, E.F.; Ober, W. Fundamentals of Anatomy and Physiology 2001; Pentice Hall: Hoboken, NJ, USA, 2015; pp. 538–557. [Google Scholar]
- Park, K.S. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine 2013, 62, 407–412. [Google Scholar] [CrossRef]
- Wang, S.-N.; Xie, G.-P.; Qin, C.-H.; Chen, Y.-R.; Zhang, K.-R.; Li, X.; Wu, Q.; Dong, W.-Q.; Yang, J.; Yu, B. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int. Immunopharmacol. 2015, 24, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kartini Piyaviriyakul, S.; Thongpraditchote, S.; Siripong, P.; Vallisuta, O. Effects of Plantago major extracts and its chemical compounds on proliferation of cancer cells and cytokines production of lipopolysaccharide-activated THP-1 macrophages. Pharmacogn. Mag. 2017, 13, 393–399. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, Z.; Zhang, J.; Ren, C.; Zhang, Q. Aucubin alleviates diabetic nephropathy by inhibiting NF-κB activation and inducing SIRT1/SIRT3-FOXO3a signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. J. Funct. Foods. 2020, 64, 103702. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, B.; Lv, L.; Wang, Z.; He, J.; Chen, Z.; Wen, X.; Zhang, Y.; Sun, W.; Li, Y.; et al. Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci. 2017, 189, 44–51. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, X.; Zong, W.; Wang, X.; Chen, L.; Zhou, L.; Li, C.; Huang, Q.; Huang, X.; Zeng, G.; et al. Aucubin alleviates seizures activity in Li-Pilocarpine-induced epileptic mice: Involvement of inhibition of neuroinflammation and regulation of neurotransmission. Neurochem. Res. 2019, 44, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, X.-M.; Wu, L.-Y.; Liu, G.-J.; Xu, W.-D.; Zhang, X.-S.; Gao, Y.-Y.; Tao, T.; Zhou, Y.; Lu, Y.; et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J. Neuroinflammation 2020, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Jung, E.; Kim, J. Aucuba japonica extract and aucubin prevent desiccating stress-induced corneal epithelial cell injury and improve tear secretion in a mouse model of dry eye disease. Molecules 2018, 23, 2599. [Google Scholar] [CrossRef] [PubMed]
- Potočnjak, I.; Marinić, J.; Batičić, L.; Šimić, L.; Broznić, D.; Domitrović, R. Aucubin administered by either oral or parenteral route protects against cisplatin-induced acute kidney injury in mice. Food Chem. Toxicol. 2020, 142, 111472. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell. Mol. Med. 2019, 23, 4063–4075. [Google Scholar] [CrossRef]
- Duan, M.X.; Yuan, Y.; Liu, C.; Cai, Z.; Xie, Q.; Hu, T.; Tang, Q.; Wu, Q.Q. Indigo fruits ingredient, aucubin, protects against LPS-induced cardiac dysfunction in mice. J. Pharmacol. Exp. Ther. 2019, 371, 348–359. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Z.; Gao, W.; Duan, Y.; Fan, G.; Geng, X.; Wu, B.; Li, K.; Liu, K.; Peng, C. Aucubin attenuates liver ischemia-reperfusion injury by inhibiting the HMGB1/TLR-4/NF-κB signaling pathway, oxidative stress, and apoptosis. Front. Pharmacol. 2020, 11, 544124. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Cheng, X.-N.; Bai, F.; Fang, L.-Y.; Hu, H.-Z.; Sun, D.-Q. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed. Pharmacother. 2018, 106, 192–199. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Lv, P.-Y.; Feng, H.; Huang, W.-H.; Tian, Y.-Y.; Wang, Y.-Q.; Qin, Y.-H.; Li, X.-H.; Hu, K.; Zhou, H.-H.; Ouyang, D.-S. Aucubin and its hydrolytic derivative attenuate activation of hepatic stellate cells via modulation of TGF-β stimulation. Environ. Toxicol. Pharmacol. 2017, 50, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xie, Q.; Huang, Y.; Zhang, S.; Chen, Y. Aucubin suppresses Titanium particles-mediated apoptosis of MC3T3-E1 cells and facilitates osteogenesis by affecting the BMP2/Smads/RunX2 signaling pathway. Mol. Med. Rep. 2018, 18, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, J.; Zhu, Z.; Bao, X.; Zhang, M.; Ren, C.; Zhang, Q. Aucubin, a natural iridoid glucoside, attenuates oxidative stress-induced testis injury by inhibiting JNK and CHOP activation via Nrf2 up-regulation. Phytomedicine 2019, 64, 153057. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, Q.-Q.; Xiao, Y.; Duan, M.X.; Liu, C.; Yuan, Y.; Meng, Y.-Y.; Liao, H.H.; Tang, Q.-Z. Aucubin protects against myocardial infarction-induced cardiac remodeling via nNOS/NO-regulated oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 4327901. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, X.; Lu, W.; Liu, X.; Hu, M.; Wang, D. Aucubin exerts anti-osteoporotic effects by promoting osteoblast differentiation. Aging 2020, 12, 2226–2245. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, Y.; Yao, Z.-L.; Chen, P.-S.; Yu, B.; Wang, S.-N. Aucubin protects chondrocytes against IL-1β-induced apoptosis in vitro and inhibits osteoarthritis in mice model. Drug Des. Dev. Ther. 2019, 13, 3529–3538. [Google Scholar] [CrossRef]
- Kaltenboeck, A.; Harmer, C. The neuroscience of depressive disorders: A brief review of the past and some considerations about the future. Brain Neurosci. Adv. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Brigitta, B. Pathophysiology of depression and mechanisms of treatment. Dialog-Clin. Neurosci. 2022, 4, 7–20. [Google Scholar] [CrossRef]
- Möhler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef]
- Rodwell, V.W. Harper’s Illustrated Biochemistry; McGraw-Hill Education: Berkshire, UK, 2015. [Google Scholar]
- Xue, H.Y.; Lu, Y.N.; Fang, X.M.; Xu, Y.P.; Gao, G.Z.; Jin, L.J. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol. Biol. Rep. 2012, 39, 9311–9318. [Google Scholar] [CrossRef]
- Jung, E.; Park, S.-B.; Jung, W.K.; Kim, H.R.; Kim, J. Antiglycation activity of aucubin in vitro and in exogenous methylglyoxal injected rats. Molecules 2019, 24, 3653. [Google Scholar] [CrossRef] [PubMed]
- Tortora, G. Principles of Anatomy and Physiology, 2012; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zhou, Y.; Li, P.; Duan, J.-X.; Liu, T.; Guan, X.-X.; Mei, W.-X.; Liu, Y.-P.; Sun, G.-Y.; Wan, L.; Zhong, W.-J.; et al. Aucubin alleviates bleomycin-induced pulmonary fibrosis in a mouse model. Inflammation 2017, 40, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Lara, A.R.; Thannickal, V.J. Lactate, a Novel Trigger of Transforming Growth Factor-β Activation in Idiopathic Pulmonary Fibrosis; American Thoracic Society: New York, NY, USA, 2012; pp. 701–703. [Google Scholar] [CrossRef]
- Bannister, B.; Gillespie, S.H.; Jones, J. Infection: Microbiology and Management; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Silva-Dias, A.; Miranda, I.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef]
- Shirley, K.P.; Windsor, L.J.; Eckert, G.J.; Gregory, R.L. In vitro effects of Plantago major extract, aucubin, and baicalein on Candida albicans biofilm formation, metabolic activity, and cell surface hydrophobicity. J. Prosthodont. 2017, 26, 508–515. [Google Scholar] [CrossRef]
- Senatore, F.; Rigano, D.; Formisano, C.; Grassia, A.; Basile, A.; Sorbo, S. Phytogrowth-inhibitory and antibacterial activity of Verbascum sinuatum. Fitoterapia 2007, 78, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, D.; Zhao, S.-Q.; Su, J.; Yan, Q.-P.; Chen, L.; Xiao, Y.; Zhang, C.-M. Enzymatic extraction and antibacterial activity of aucubin from Eucommia ulmoides leaves. Zhong Yao Cai 2012, 35, 304–306. [Google Scholar] [PubMed]
- Shattat, G.F. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed. Pharmacol. J. 2015, 7, 399–409. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Kim, M.-B.; Kim, C.; Chung, W.-S.; Cho, J.-H.; Nam, D.; Kim, S.-H.; Ahn, K.S. The Hydrolysed Products of Iridoid Glycosides Can Enhance Imatinib Mesylate-Induced Apoptosis in Human Myeloid Leukaemia Cells. Phytother. Res. 2015, 29, 434–443. [Google Scholar] [CrossRef]
- Wu, Q.-Q.; Xiao, Y.; Duan, M.-X.; Yuan, Y.; Jiang, X.-H.; Yang, Z.; Liao, H.-H.; Deng, W.; Tang, Q.-Z. Aucubin protects against pressure overload-induced cardiac remodelling via the β3-adrenoceptor–neuronal NOS cascades. Br. J. Pharmacol. 2018, 175, 1548–1566. [Google Scholar] [CrossRef]
- Kim, Y.M.; Sim, U.-C.; Shin, Y.; Kwon, Y.S.A.Y.K. Aucubin promotes neurite outgrowth in neural stem cells and axonal regeneration in sciatic nerves. Exp. Neurobiol. 2014, 23, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.-M.; Chang, K.-S.; YunChoi, H.-S. Toxicological Studies on Aucubin (I)-Acute Toxicities and Effects on Blood Serum Enzymes. Kor. J. Pharmacog. 1983, 14, 95–101. [Google Scholar]
- Chang, I.-M.; Chang, K.-S.; Yun-Choi, H.S. Pharmacology and toxicology of aucubin. Yakhak Hoeji 1984, 28, 35–48. [Google Scholar]



| Family | Species | Part of Plant | Reference |
|---|---|---|---|
| Cornaceae | Aucuba japonica | Leaves | [14] |
| Eucommiaceae | Eucommia ulmoides | Seeds, fruits | [15,16,17] |
| Buddlejaceae | Buddleja globosa | Leaves | [18] |
| Buddleja asiatica | Aerial flowering parts | [19] | |
| Lamiaceae | Vitex agnus-castus | Flowers, leaves, twigs | [20] |
| Lentibulariaceae | Utricularia australis | N/A | [21] |
| Orobanchaceae | Bellardia trixago | Aerial parts | [22] |
| Castilleja tenuiflora | Aerial parts | [23] | |
| Centranthera grandiflora | Roots, stems, leaves, flowers | [24] | |
| Melampyrum arvense | Aerial parts | [25] | |
| Parentucellia viscosa | Whole plants | [26] | |
| Rehmannia glutinosa | Roots, leaves | [27] | |
| Plantaginaceae | Aragoa cundinamarcensis | Aerial parts | [28] |
| Campylanthus salsaloides | Aerial parts | [29] | |
| Campylanthus glaber | Aerial parts | [29] | |
| Globularia alypum | Leaves, flowers, woody stems, underground parts | [30] | |
| Globularia dumulosa | Aerial parts | [31] | |
| Globularia cordifolia | Roots, rhizomes | [30,32] | |
| Globularia meridionalis | Leaves, flowers, woody stems, underground parts | [30] | |
| Globularia punctata | Leaves, flowers, woody stems, underground parts | [30] | |
| Linaria alpina | Aerial parts | [33] | |
| Paederota lutea | Whole plants | [34] | |
| Plantago lanceolata | Aerial parts | [35] | |
| Plantago lagopus | Aerial parts | [36] | |
| Plantago major | Aerial parts | [37] | |
| Plantago myosuros | Whole plants | [38] | |
| Veronica beccabunga | Leaves | [39] | |
| Veronica hookeri | N/A | [40] | |
| Veronica pectinata | Aerial parts | [41] | |
| Veronica pinguifolia | N/A | [40] | |
| Scrophulariaceae | Scrophularia nodosa | Leaves, flowers, stems, roots | [42] |
| Sutera dissecta | Aerial parts | [43] | |
| Verbascum lasianthum | Flowers, roots | [10,44] | |
| Verbascum macrurum | Aerial parts | [45] | |
| Verbascum mucronatum | Flowers | [46] |
| No | Plant and Plant Part | Extraction Method and Solvent | Isolation Method | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Eucommia ulmoides; seeds | Smashing tissue extraction using methanol | The crude extract was defatted using petroleum ether; then, column chromatography of the residue was conducted using Si gel as stationary phase and petroleum ether-EtOAc (50:1 to 1:10) as the mobile phase, followed by another column chromatography using Sephadex LH-20 as the stationary phase and petroleum ether-EtOAc (1:8) as the mobile phase. | 0.28 | [16] |
| 2 | Plantago major; aerial parts | Maceration using methanol | The crude extract was partitioned using dichloroethane-H2O; the water-soluble part was then cleaned using charcoal, followed by CC using stationary phase C-18 and different mobile phases: H2O, H2O-MeOH (95:5, 70:30, 50:50), MeOH, MeOH-Me2CO (1:1), and MeOH-Cl(CH2)2Cl (1:1). Then, the MeOH-Cl(CH2)2Cl (1:1) fraction was purified with Si gel. | 0.055 | [37] |
| 3 | Campylanthus salsaloides; dried and fresh aerial parts | Boiling in ethanol for 5 min, followed by 6 d of maceration | The crude extract was partitioned in Et2O-H2O; the aqueous phase was then evaporated and treated with charcoal, followed by reversed-phase CC (C-size Lobar®) using mobile phase H2O-MeOH (1:0 to 2:1). | 0.15 (dried aerial parts), 0.32 (fresh aerial parts) | [29] |
| 4 | Globularia dumulosa; aerial parts | Digestion using methanol at 45 °C | The crude extract was partitioned in H2O-CHCl3; then, the water fraction was lyophilized, followed by VLC with stationary phase C-18 and different mobile phases: H2O, H2O-MeOH (5–80% MeOH in H2O), and MeOH. The subsequent VLC used Si gel as the stationary phase and CHCl3-MeOH-H2O (90:10:1 to 50:50:5) as the mobile phase, followed by MPLC using stationary phase C-18 and mobile phase MeOH in water (0–40%). | 0.079 | [31] |
| 5 | Aragoa cundinamarcensis; aerial parts | Boiling in EtOH, followed by maceration for 3 d | The crude extract was partitioned in Et2O-H2O; the aqueous phase was then cleaned using activated carbon in MeOH, followed by CC using stationary phase C-18 and mobile phase H2O-MeOH (1:0 to 2:1). | 1.7 | [28] |
| 6 | Verbascum lasianthum; roots | Digestion using methanol at 40 °C | The crude extract was partitioned in CHCl3-H2O; the aqueous phase was then lyophilized, followed by CC using polyamide as the stationary phase and H2O and an H2O-MeOH mixture as the mobile phase. Then, VLC was conducted using C-18 as the stationary phase and H2O-MeOH as the mobile phase (0–100%, gradient). | 0.06 | [10] |
| 7 | Verbascum mucronatum; flowers | Digestion using methanol at 40 °C | The crude extract was partitioned in CHCl3-H2O, followed by CC using polyamide as the stationary phase and H2O and an H2O-MeOH mixture as the mobile phase. Then, VLC using stationary phase C-18 and gradient mobile phase H2O-MeOH (0–100%) was conducted. | 0.02 | [46] |
| 8 | Plantago myosuros; whole plants, frozen | Maceration using ethanol | The crude extract was partitioned using Et2O-H2O; the aqueous phase was then cleaned using activated carbon in MeOH, followed by CC using stationary phase Lobar RP18 and mobile phase H2O-MeOH (25:1 to 1:1). | 0.04 | [38] |
| 9 | Eucommia ulmoides; fruits | UAE using 0.5 mol/L ([Bmim]Br) ionic liquid | The ionic liquid extract was placed onto a glass column containing HPD850 resins; then, the column was washed using deionized water and eluted (desorption) using 10–80% EtOH. This process ended with the vacuum distillation of the eluent, 40–80% ethanol. | N/A | [17] |
| 10 | Globularia cordifolia; roots and rhizomes | Digestion using methanol at 45 °C | The crude extract was partitioned using H2O-CHCl3; then, the aqueous phase was lyophilized, followed by VLC using LiChroprep C-18 as the stationary phase and H2O and a mixture of H2O-MeOH (10–90% MeOH) as the mobile phase. The subsequent MPLC was performed using C-18 as the stationary phase and MeOH in H2O (0–50%, MeOH) as the mobile phase, followed by CC using stationary phase Si gel and mobile phase CH2Cl2-MeOH-H2O (70:30:3). | 0.004 | [32] |
| 11 | Bellardia trixago; aerial parts | Remaceration using methanol | The crude extract was partitioned using water–petroleum ether, followed by chloroform and n-butanol. The butanol fraction was then column-chromatographed using stationary phase Si gel and mobile phase CHCl3-MeOH-H2O (80:20:1, 80:20:2, to 50:50:5). Fraction D underwent MPLC using 15–25% MeOH as the mobile phase. | 0.06 | [51] |
| 12 | Veronica pectinata L. Var. glandulosa; aerial parts | Digestion using MeOH at 40 °C | The crude extract was partitioned using water-CHCl3. The water fraction was then lyophilized, followed by CC using stationary phase polyamide and mobile phase H2O-MeOH, made in gradients by increasing the MeOH concentration to produce five fractions. Fraction A was then chromatographed using stationary phase Si gel and mobile phase CHCl3:MeOH:H2O (90:10:1 to 60:40:4), followed by MPLC using stationary phase RP-18 and gradient mobile phase MeOH (20–50%). | 0.027 | [41] |
| 13 | Paederota lutea; whole plants | Brought to a boil using EtOH, followed by 7 d of maceration | The crude extract was partitioned using H2O-Et2O. The water fraction was then chromatographed using stationary phase RP-18 and mobile phase H2O-MeOH (25:1 to 1:1). | 0.349 | [34] |
| 14 | Vitex agnus-castus; flowers, leaves, and twigs | Digestion using MeOH at 45 °C | The crude extract was partitioned using H2O-CHCl3, followed by n-BuOH. The n-BuOH fraction was then column-chromatographed using stationary phase Si gel and mobile phase CHCl3 (by increasing MeOH gradually). Further separation and purification were conducted by CC using Si gel as the stationary phase and EtOAc:MeOH:H2O (100:5:2 to 100:17:13) and CHCl3:MeOH:H2O (90:10:1 to 60:40:4) as mobile phases, CC using stationary phase Sephadex LH-20 and mobile phase MeOH, and CC using stationary phase RP-18 and mobile phase MeOH in H2O (made in gradients). | 0.006 | [20] |
| 15 | Verbascum lasianthum; flowers | Digestion using MeOH at 40 °C | The crude extract was partitioned using H2O-CHCl3. The water phase was lyophilized and then processed with VLC using polyamide as the stationary phase and H2O as the mobile phase (with increasing MeOH concentrations), VLC using stationary phase C-18 and mobile phase H2O-MeOH (0–100% MeOH). Further separation was conducted by CC using Si gel as the stationary phase and CHCl3, CHCl3:MeOH (95:5), and CHCl3:MeOH:H2O (70:30:3) as mobile phases, and VLC with C-18 as the stationary phase and H2O and gradient MeOH-H2O (0–20% MeOH) as mobile phases. | 0.028 | [44] |
| 16 | Castilleja tenuiflora; aerial parts | Maceration using ethanol | The crude extract was partitioned using H2O-Et2O. The H2O phase was then concentrated, dissolved in MeOH, and cleaned using activated carbon. Further separation was carried out using CC (stationary phase Si gel and mobile phase hexane-CH2Cl2-AcOEt-MeOH with increasing polarity) and MPLC (C-18 as the stationary phase, H2O:MeOH (10:0 to 1:1) as the mobile phase). | 0.46 | [23] |
| 17 | Plantago lagopus; aerial parts | Digestion using MeOH at 40 °C | The crude extract was partitioned using water–petroleum ether. The H2O phase was column-chromatographed (polyamide as the stationary phase, 0–100% MeOH as the mobile phase). The water fraction was then extracted using n-butanol, followed by MPLC and CC (Si gel as the stationary phase; CHCL3:MeOH at 100:0, 95:5, 90:10, 85:15, 80:20; and 75:25 as the mobile phase). | N/A | [36] |
| 18 | Parentucellia viscosa; whole plants | Remaceration using 96% EtOH | The crude extract was column-chromatographed using Si gel as the stationary phase and n-butanol saturated with water and CHCl3/MeOH at various ratios as the mobile phase. | 0.16 | [26] |
| 19 | Veronica beccabunga; leaves | Brought to boil using EtOH | The crude extract was partitioned using Et2O-H2O; then, the H2O phase was dried and dissolved in 10% acetic acid. The aliquots were then column-chromatographed with stationary phase RP-18 and mobile phase H2O-MeOH (1:0 to 1:1). | 0.25 | [39] |
| 20 | Veronica hookeri and Veronica pinguifolia; N/A | Maceration using MeOH | The crude extract was partitioned using Et2O-H2O; then, the H2O phase was dried and column-chromatographed with stationary phase RP-18 and mobile phase H2O-MeOH (25:1 to 1:1). | 0.18 (V. hookeri), 0.08 (V. pinguifolia) | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartini, K.; Irawan, M.A.; Setiawan, F.; Jayani, N.I.E. Characteristics, Isolation Methods, and Biological Properties of Aucubin. Molecules 2023, 28, 4154. https://doi.org/10.3390/molecules28104154
Kartini K, Irawan MA, Setiawan F, Jayani NIE. Characteristics, Isolation Methods, and Biological Properties of Aucubin. Molecules. 2023; 28(10):4154. https://doi.org/10.3390/molecules28104154
Chicago/Turabian StyleKartini, Kartini, Michelle Abigail Irawan, Finna Setiawan, and Nikmatul Ikhrom Eka Jayani. 2023. "Characteristics, Isolation Methods, and Biological Properties of Aucubin" Molecules 28, no. 10: 4154. https://doi.org/10.3390/molecules28104154