Composition and Antifungal Activity of the Alkaloidal Fraction of Lupinus mirabilis Leaves: A Biochemometrics-Based Exploration
Abstract
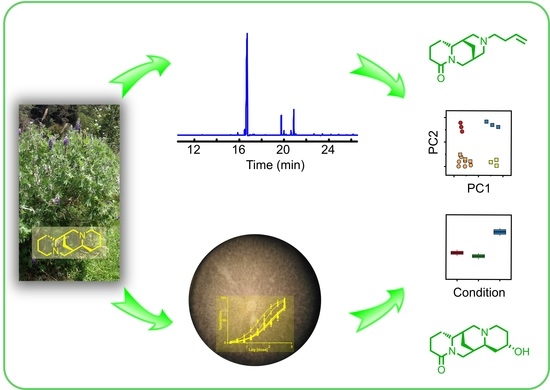
:1. Introduction
2. Results and Discussion
2.1. Analysis of Alkaloidal Composition
2.2. Mycelial Growth Inhibition
2.3. Variation of Chemical Composition and Mycelial Growth Inhibition by Extraction Conditions: A Biochemometrics-Based Exploration
3. Materials and Methods
3.1. Plant Material
3.2. Conventional Extraction
3.3. Ultrasound-Assisted Extraction
3.4. GC-MS Analysis
3.5. Mycelial Growth Inhibition Assay
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| # a | RT b | RI c | M+ d | Other MS Fragments d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.87 | 1757 | 234(39) | 98(100) | 137(55) | 136(36) | 97(25) | 110(23) | 84(20) | 193(17) | 122(16) | 134(15) | 150(15) |
| 2 | 16.73 | 1786 | 234(26) | 137(100) | 98(87) | 136(42) | 97(34) | 193(33) | 110(22) | 122(19) | 84(18) | 150(15) | 134(15) |
| 3 | 16.93 | 1852 | 234(19) | 137(100) | 98(95) | 136(43) | 97(40) | 193(28) | 110(24) | 122(21) | 84(20) | 134(18) | 150(15) |
| 4 | 17.24 | 1887 | 234(15) | 98(100) | 137(90) | 134(46) | 97(43) | 122(26) | 232(26) | 110(24) | 84(19) | 150(17) | 193(16) |
| 5 | 17.62 | 1920 | 232(37) | 98(100) | 137(62) | 97(44) | 134(39) | 84(29) | 122(22) | 110(19) | 148(16) | 193(15) | 177(12) |
| 6 | 17.75 | 1934 | 208(47) | 165(100) | 110(99) | 136(88) | 123(85) | 191(78) | 137(73) | 94(69) | 95(45) | 84(40) | 107(29) |
| 7 | 19.75 | 2133 | 248(<1) | 58(100) | 207(87) | 112(31) | 108(16) | 208(12) | 55(18) | 94(6) | 84(4) | 98(3) | - |
| 8 | 19.99 | 2172 | 248(51) | 97(100) | 98(87) | 110(73) | 136(49) | 220(35) | 123(33) | 137(28) | 150(23) | 134(22) | 191(16) |
| 9 | 20.36 | 2204 | 248(44) | 136(100) | 149(52) | 98(38) | 150(32) | 97(31) | 110(23) | 137(22) | 84(20) | 134(18) | 122(13) |
| 10 | 20.63 | 2236 | 264(<1) | 223(100) | 58(68) | 108(60) | 96(20) | 128(18) | 224(14) | 100(9) | 110(6) | 82(6) | - |
| 11 | 20.88 | 2275 | 248(44) | 136(100) | 149(54) | 150(36) | 98(26) | 110(22) | 134(20) | 137(15) | 82(10) | 219(7) | - |
| 12 | 23.09 | 2531 | 264(30) | 152(100) | 134(50) | 246(50) | 165(38) | 112(36) | 148(26) | 98(17) | 122(15) | 84(14) | 207(12) |
| 13 | 24.85 | 2623 | 337(3) | 59(100) | 72(67) | 55(41) | 43(28) | 126(14) | 86(10) | 114(8) | 140(4) | 320(3) | 294(2) |

References
- Drummond, C.S.; Eastwood, R.J.; Miotto, S.T.S.; Hughes, C.E. Multiple Continental Radiations and Correlates of Diversification in Lupinus (Leguminosae): Testing for Key Innovation with Incomplete Taxon Sampling. Syst. Biol. 2012, 61, 443–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aïnouche, A.-K.; Bayer, R.J. Phylogenetic Relationships in Lupinus (Fabaceae: Papilionoideae) Based on Internal Transcribed Spacer Sequences (ITS) of Nuclear Ribosomal DNA. Am. J. Bot. 1999, 86, 590. [Google Scholar] [CrossRef] [PubMed]
- Aïnouche, A.; Bayer, R.J.; Misset, M.-T. Molecular Phylogeny, Diversification and Character Evolution in Lupinus (Fabaceae) with Special Attention to Mediterranean and African Lupines. Plant Syst. Evol. 2004, 246, 211–222. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.E.; Jayasena, V. Phytochemical Composition and Bioactivities of Lupin: A Review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. Nutritional, Health, and Technological Functionality of Lupin Flour Addition to Bread and Other Baked Products: Benefits and Challenges. Crit. Rev. Food Sci. Nutr. 2016, 56, 835–857. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Murakoshi, I. Chemistry, Biochemistry and Chemotaxonomy of Lupine Alkaloids in the Leguminosae. Stud. Nat. Prod. Chem. 1995, 15, 519–549. [Google Scholar] [CrossRef]
- Reisfeld, R.A.; Jolla, L.; Tuppy, H. Lupine Alkaloids. In Molecular Biology, Biochemistry, and Biophysics—The Biochemistry of Alkaloids; Springer: Berlin, Germany, 1980; Volume 32, pp. 67–75. ISBN 9783642618321. [Google Scholar]
- Frick, K.M.; Kamphuis, L.G.; Siddique, K.H.M.; Singh, K.B.; Foley, R.C. Quinolizidine Alkaloid Biosynthesis in Lupins and Prospects for Grain Quality Improvement. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Schrenk, D.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; Nielsen, E.; et al. Scientific Opinion on the Risks for Animal and Human Health Related to the Presence of Quinolizidine Alkaloids in Feed and Food, in Particular in Lupins and Lupin-Derived Products. Eur. Food Saf. Auth. J. 2019, 17, e05860. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Avendaño, P.; Tarvainen, M.; Suomela, J.P.; Glorio-Paulet, P.; Yang, B.; Repo-Carrasco-Valencia, R. Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus mutabilis Sweet after Aqueous Debittering Process. Plant Foods Hum. Nutr. 2020, 75, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Introduction: Biochemistry, Physiology and Ecological Functions of Secondary Metabolites. In Biochemistry of Plant Secondary Metabolism; Wiley-Blackwell: Oxford, UK, 2010; Volume 40, pp. 1–19. ISBN 9781444320503. [Google Scholar]
- Boschin, G.; Resta, D. Alkaloids Derived from Lysine: Quinolizidine (a Focus on Lupin Alkaloids) BT—Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. In Natural Products. Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 381–403. ISBN 978-3-642-22144-6. [Google Scholar]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction ? Front. Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Confortin, T.C.; Todero, I.; Soares, J.F.; Luft, L.; Brun, T.; Rabuske, J.E.; Nogueira, C.U.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extracts from Lupinus albescens: Antioxidant Power and Antifungal Activity in vitro against Phytopathogenic Fungi. Environ. Technol. 2019, 40, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Alcocer, A.; Zamora-Natera, J.F.; Virgen-calleros, G.; Nuño-romero, R. In Vitro Biological Activity of Extracts of Lupinus Spp. on Phytopathogenic Fungi. Rev. Mex. Fitopatol. 2005, 23, 140–146. [Google Scholar]
- Small, E. 38. Lupins—Benefit and Harm Potentials. Biodiversity 2012, 13, 54–64. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The Epidemiology of Fusarium Wilt of Banana. Front. Plant Sci. 2019, 10, 1–53. [Google Scholar] [CrossRef] [Green Version]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Álvarez-Caballero, J.M.; Cuca-Suárez, L.E.; Coy-Barrera, E. Bio-Guided Fractionation of Ethanol Extract of Leaves of Esenbeckia alata Kunt (Rutaceae) Led to the Isolation of Two Cytotoxic Quinoline Alkaloids: Evidence of Selectivity against Leukemia Cells. Biomolecules 2019, 9, 585. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Nunes, D.S.; Barba, F.J. An Integrated Strategy between Food Chemistry, Biology, Nutrition, Pharmacology, and Statistics in the Development of Functional Foods: A Proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Todd, D.A.; Egan, J.M.; Raja, H.A.; Oberlies, N.H.; Kvalheim, O.M.; Cech, N.B. Biochemometrics for Natural Products Research: Comparison of Data Analysis Approaches and Application to Identification of Bioactive Compounds. J. Nat. Prod. 2016, 79, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas-Laverde, D.; Barbosa-Cornelio, R.; Coy-Barrera, E. Antifungal Activity against Fusarium oxysporum of Botanical End-Products: An Integration of Chemical Composition and Antifungal Activity Datasets to Identify Antifungal Bioactives. Plants 2021, 10, 2563. [Google Scholar] [CrossRef]
- Kellogg, J.J.; Wallace, E.D.; Graf, T.N.; Oberlies, N.H.; Cech, N.B. Conventional and Accelerated-Solvent Extractions of Green Tea (Camellia sinensis) for Metabolomics-Based Chemometrics. J. Pharm. Biomed. Anal. 2017, 145, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Caesar, L.K.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Opportunities and Limitations for Untargeted Mass Spectrometry Metabolomics to Identify Biologically Active Constituents in Complex Natural Product Mixtures. J. Nat. Prod. 2019, 82, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Chemische Verteidigung Der Lupinen: Zur Biologischen Bedeutung Der Chinolizidinalkaloide. Plant Syst. Evol. 1985, 150, 65–81. [Google Scholar] [CrossRef]
- Priddis, C.R. Capillary Gas Chromatography of Lupin Alkaloids. J. Chromatogr. A 1983, 261, 95–101. [Google Scholar] [CrossRef]
- Wink, M.; Meißner, C.; Witte, L. Patterns of Quinolizidine Alkaloids in 56 Species of the Genus Lupinus. Phytochemistry 1995, 38, 139–153. [Google Scholar] [CrossRef]
- Wink, M. Quinolizidine Alkaloids: Biochemistry, Metabolism, and Function in Plants and Cell Suspension Cultures. Planta Med. 1987, 53, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Vul, N.S.; Zaikin, V.G. Mass Spectrometry of Quinolizidine Alkaloids. Russ. Chem. Rev. 1976, 45, 959–970. [Google Scholar]
- El-Shazly, A.; Ateya, A.-M.M.M.; Wink, M. Quinolizidine Alkaloid Profiles of Lupinus varius orientalis, L. albus albus, L. hartwegii, and L. densiflorus. Z. Naturforsch. C 2001, 56, 21–30. [Google Scholar] [CrossRef]
- Planchuelo-Ravelo, A.; Witte, L.; Wink, M. Quinolizidine Alkaloid Profiles of South American Lupins: Lupinus linearis and the Lupinus gibertianus Complex. Z. Naturforsch. C 1993, 48c, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Chludil, H.D.; Vilariño, M.D.P.; Franco, M.L.; Leicach, S.R. Changes in Lupinus albus and Lupinus angustifolius Alkaloid Profiles in Response to Mechanical Damage. J. Agric. Food Chem. 2009, 57, 6107–6113. [Google Scholar] [CrossRef]
- Kordan, B.; Dancewicz, K.; Wróblewska, A.; Gabryś, B. Intraspecific Variation in Alkaloid Profile of Four Lupine Species with Implications for the Pea Aphid Probing Behaviour. Phytochem. Lett. 2012, 5, 71–77. [Google Scholar] [CrossRef]
- Cook, D.; Mott, I.W.; Larson, S.R.; Lee, S.T.; Johnson, R.; Stonecipher, C.A.; Herbarium, S.L.W. Genetic Relationships among Different Chemotypes of Lupinus sulphureus. J. Agric. Food Chem. 2018, 66, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Cely-Veloza, W.; Quiroga, D.; Coy-Barrera, E. Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules 2022, 27, 305. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laínez, D.; García-Mateos, R.; San Miguel-Chávez, R.; Soto-Hernández, M.; Rodríguez-Pérez, E.; Kite, G. Bactericidal and Fungicidal Activities of Calia secundiflora (Ort.) Yakovlev. Z. Naturforsch. C 2008, 63, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Chemical Defense of Leguminosae. Are Quinolizidine Alkaloids Part of the Antimicrobial Defense System of Lupins? Z. Naturforsch. C 1984, 39, 548–552. [Google Scholar] [CrossRef] [Green Version]
- El Hamdani, N.; Filali-Ansari, N.; Fdil, R.; El Abbouyi, A.; El Khyari, S. Antifungal Activity of the Alkaloids Extracts from Aerial Parts of Retama monosperma. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 965–971. [Google Scholar]
- Marentes-Culma, R.; Orduz-Díaz, L.L.; Coy-Barrera, E. Targeted Metabolite Profiling-Based Identification of Antifungal 5-n-Alkylresorcinols Occurring in Different Cereals against Fusarium oxysporum. Molecules 2019, 24, 770. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]





| Compound | Relative Abundance (%) a | |||||
|---|---|---|---|---|---|---|
| CSE1 | CSE2 | CSE3 | UAE1 | UAE2 | UAE3 | |
| 1 | 0.3 ± 0.1 C | 0.7 ± 0.1 B | 0.7 ± 0.1 B | 0.3 ± 0.1 C | 1.1 ± 0.1 A | 0.5 ± 0.03 B,C |
| 2 | 74.3 ± 1.5 B | 80.4 ± 1.7 A | 80.6 ± 2.1 A | 79.6 ± 1.1 A | 74.4 ± 1.3 B | 64.7 ± 1.7 C |
| 3 | - | 0.8 ± 0.1 B | 0.8 ± 0.1 B | 0.8 ± 0.1 B | 1.3 ± 0.1 A | - |
| 4 | - | - | 0.6 ± 0.1 B | 0.2 ± 0.1 C | 1.2 ± 0.1 A | 0.7 ± 0.1 B |
| 5 | - | - | - | - | 0.4 ± 0.1 A | - |
| 6 | - | 0.5 ± 0.1 B | 0.03 ± 0.01 D | 0.3 ± 0.1 C | 0.7 ± 0.1 A | - |
| 7 | 1.6 ± 0.2 D | 3.0 ± 0.2 B,C | 2.7 ± 0.2 C | 3.5 ± 0.2 B | 3.6 ± 0.2 B | 4.9 ± 0.3 A |
| 8 | 0.4 ± 0.1 D | 1.5 ± 0.1 A,B | 1.8 ± 0.2 A | 1.0 ± 0.1 C | 1.0 ± 0.1 C | 1.4 ± 0.1 B |
| 9 | - | 0.3 ± 0.1 B | 0.2 ± 0.1 B | 0.3 ± 0.1 B | 0.3 ± 0.1 B | 0.6 ± 0.1 A |
| 10 | 0.6 ± 0.1 E | 0.9 ± 0.1 C,D | 0.8 ± 0.1 D,E | 1.2 ± 0.1 B | 1.1 ± 0.1 B,C | 1.6 ± 0.1 A |
| 11 | 4.5 ± 0.3 C | 7.9 ± 0.5 B | 7.7 ± 0.5 B | 10.0 ± 0.6 A | 9.9 ± 0.5 A | 10.4 ± 0.5 A |
| 12 | - | 0.4 ± 0.1 C | 0.8 ± 0.1 B | - | 1.0 ± 0.1 B | 1.7 ± 0.2 A |
| 13 | - | 0.4 ± 0.1 A,B | 0.2 ± 0.1 B,C | 0.4 ± 0.1 A,B | 0.2 ± 0.1 B,C | 0.6 ± 0.1 A |
| Total b | 82.0 ± 2.1 B | 96.8 ± 2.7 A | 97.4 ± 2.3 A | 97.7 ± 1.2 A | 96.4 ± 1.5 A | 95.6 ± 1.8 A |
| IC50 (µg/mL) | Confidence Interval (95%) | |
|---|---|---|
| CSE1 | 98.5 E | 90.1–102.1 |
| CSE2 | 91.3 DE | 84.3–93.7 |
| CSE3 | 84.8 D | 80.0–87.3 |
| UAE1 | 86.3 DE | 84.5–92.8 |
| UAE2 | 62.3 C | 56.9–66.2 |
| UAE3 | 33.5 B | 27.5–35.6 |
| P a | 17.7 A | 15.5–20.6 |
| M a | 11.9 A | 7.7–14.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, F.A.; Coy-Barrera, E. Composition and Antifungal Activity of the Alkaloidal Fraction of Lupinus mirabilis Leaves: A Biochemometrics-Based Exploration. Molecules 2022, 27, 2832. https://doi.org/10.3390/molecules27092832
Bernal FA, Coy-Barrera E. Composition and Antifungal Activity of the Alkaloidal Fraction of Lupinus mirabilis Leaves: A Biochemometrics-Based Exploration. Molecules. 2022; 27(9):2832. https://doi.org/10.3390/molecules27092832
Chicago/Turabian StyleBernal, Freddy A., and Ericsson Coy-Barrera. 2022. "Composition and Antifungal Activity of the Alkaloidal Fraction of Lupinus mirabilis Leaves: A Biochemometrics-Based Exploration" Molecules 27, no. 9: 2832. https://doi.org/10.3390/molecules27092832