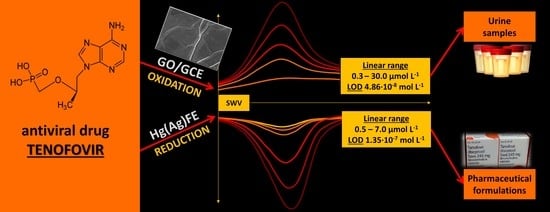
Highly Sensitive Determination of Tenofovir in Pharmaceutical Formulations and Patients Urine—Comparative Electroanalytical Studies Using Different Sensing Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties and Electrochemical Performance of the GO/GC Electrode
2.2. Electrochemical Behavior of Tenofovir
2.3. Analytical Studies
2.3.1. Calibration Curve
2.3.2. Analysis of Pharmaceutical Formulations
2.3.3. Analysis of Urine Samples
2.3.4. Selectivity of the Method
3. Materials and Methods
3.1. Apparatus
3.2. Measurement Procedure
3.3. Materials and Solutions
3.4. Analysis of Pharmaceutical Formulations and Urine Samples
3.5. Interference Studies
3.6. Validation of the Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgement
Conflicts of Interest
Sample Availability
References
- Kabbara, W.K.; Ramadan, W.H. Emtricitabine/rilpivirine/tenofovir disoproxil fumarate for the treatment of HIV-1 infection in adults. J. Infect. Public Health 2015, 8, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Travers, K.; Mboup, S.; Marlink, R.; Gueye-Nidaye, A.; Siby, T.; Thior, I.; Traore, I.; Dieng-Sarr, A.; Sankale, J.L.; Mullins, C.; et al. Natural protection against HIV-1 infection provided by HIV-2. Science 1995, 268, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.K.; Kumar, P.; Kilaru, N.B. High throughput LC-MS/MS method for simultaneous determination of tenofovir, lamivudine and nevirapine in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 931, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Pinto Neto, L.F.D.S.; Bassetti, B.R.; Fraga, I.H.V.; Oliveira Santos, C.R.; Ximenes, P.D.; Miranda, A.E. Nephrotoxicity during tenofovir treatment: A three-year follow-up study in a Brazilian reference clinic. Braz. J. Infect. Dis. 2016, 20, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezk, N.L.; Crutchley, R.D.; Kashuba, A.D. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 822, 201–208. [Google Scholar] [CrossRef]
- Sentenac, S.; Fernandez, C.; Thuillier, A.; Lechat, P.; Aymard, G. Sensitive determination of tenofovir in human plasma samples using reversed-phase liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 793, 317–324. [Google Scholar] [CrossRef]
- Shah, S.A.; Mullin, R.; Jones, G.; Shah, I.; Barker, J.; Petroczi, A.; Naughton, D.P. Simultaneous analysis of antiretroviral drugs abacavir and tenofovir in human hair by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 308–313. [Google Scholar] [CrossRef]
- Simiele, M.; Carcieri, C.; De Nicolò, A.; Ariaudo, A.; Sciandra, M.; Calcagno, A.; Bonora, S.; Di Perri, G.; D’Avolio, A. A LC–MS method to quantify tenofovir urinary concentrations in treated patients. J. Pharm. Biomed. Anal. 2015, 114, 8–11. [Google Scholar] [CrossRef]
- Sparidans, R.W.; Crommentuyn, K.M.; Schellens, J.H.; Beijnen, J.H. Liquid chromatographic assay for the antiviral nucleotide analogue tenofovir in plasma using derivatization with chloroacetaldehyde. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 791, 227–233. [Google Scholar] [CrossRef]
- Chihava, R.; Apath, D.; Moyo, M.; Shumba, M.; Chitsa, V.; Tshuma, P. One-Pot Synthesized Nickel-Cobalt Sulfide-Decorated Graphene Quantum Dot Composite for Simultaneous Electrochemical Determination of Antiretroviral Drugs: Lamivudine and Tenofovir Disoproxil Fumarate. J. Sens. 2020, 2020, 3124102. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, R. Cathodic Adsorptive Stripping Voltammetric Detection and Quantification of the Antiretroviral Drug Tenofovir in Human Plasma and a Tablet Formulation. J. Electrochem. Soc. 2013, 160, H489–H493. [Google Scholar] [CrossRef]
- Morawska, K.; Popławski, T.; Ciesielski, W.; Smarzewska, S. Electrochemical and spectroscopic studies of the interaction of antiviral drug Tenofovir with single and double stranded DNA. Bioelectrochemistry 2018, 123, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ozcelikay, G.; Dogan-Topal, B.; Ozkan, S.A. An Electrochemical Sensor Based on Silver Nanoparticles-Benzalkonium Chloride for the Voltammetric Determination of Antiviral Drug Tenofovir. Electroanalysis 2018, 30, 943–954. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Dogan-Topal, B.; Ozkan, S.A. Electrochemical characteristics of tenofovir and its determination in dosage form by electroanalytical methods. Rev. Roum. Chim. 2017, 62, 569–578. [Google Scholar]
- Barek, J.; Fischer, J.; Navrátil, T.; Pecková, K.; Yosypchuk, B.; Zima, J. Nontraditional Electrode Materials in Environmental Analysis of Biologically Active Organic Compounds. Electroanalysis 2007, 19, 2003–2014. [Google Scholar] [CrossRef]
- Mikkelsen, Ø.; Schrøder, K.H. Amalgam Electrodes for Electroanalysis. Electroanalysis 2003, 15, 679–687. [Google Scholar] [CrossRef]
- Baś, B.; Kowalski, Z. Preparation of Silver Surface for Mercury Film Electrode of Prolonged Analytical Application. Electroanalysis 2002, 14, 1067–1071. [Google Scholar] [CrossRef]
- Kowalski, Z.; A Method for Preparation of Thin Film Mercury Electrode and a Device for Preparation of Thin Film Mercury Electrode. PL 183763 B1. 31 July 2002. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.319984 (accessed on 17 March 2022).
- Baś, B.; Baś, S. Rapidly renewable silver amalgam annular band electrode for voltammetry and polarography. Electrochem. Commun. 2010, 12, 816–819. [Google Scholar] [CrossRef]
- Bas, B.; Jakubowska, M.; Gorski, L. Application of renewable silver amalgam annular band electrode to voltammetric determination of vitamins C, B1 and B2. Talanta 2011, 84, 1032–1037. [Google Scholar] [CrossRef]
- Piech, R.; Bugajna, A.; Baś, S.; Kubiak, W.W. Ultrasensitive determination of tungsten(VI) on pikomolar level in voltammetric catalytic adsorptive catechol-chlorate(V) system. J. Electroanal. Chem. 2010, 644, 74–79. [Google Scholar] [CrossRef]
- Guziejewski, D.; Morawska, K.; Popławski, T.; Metelka, R.; Ciesielski, W.; Smarzewska, S. Lactofen—Electrochemical Sensing and Interaction with dsDNA. Electroanalysis 2018, 30, 94–100. [Google Scholar] [CrossRef]
- Smarzewska, S.; Guziejewski, D.; Leniart, A.; Ciesielski, W. Nanomaterials vs. amalgam in electroanalysis: Comparative electrochemical studies of lamotrigine. J. Electrochem. Soc. 2017, 164, B321–B329. [Google Scholar] [CrossRef]
- Smarzewska, S.; Guziejewski, D.; Skowron, M.; Skrzypek, S.; Ciesielski, W. Voltammetric behavior and quantitative determination of ambazone concentrations in urine and in a pharmaceutical formulation. Cent. Eur. J. Chem. 2014, 12, 1239–1245. [Google Scholar] [CrossRef]
- De Souza Vieira, L. A review on the use of glassy carbon in advanced technological applications. Carbon 2022, 186, 282–302. [Google Scholar] [CrossRef]
- Boonmee, W.; Samoson, K.; Yodrak, J.; Thiagchanya, A.; Phonchai, A.; Limbut, W. Adsorptive Cathodic Stripping Voltammetry for Quantification of Alprazolam. Molecules 2021, 26, 2958. [Google Scholar] [CrossRef]
- Jashari, G.; Musliu, A.; Sýs, M.; Arbneshi, T.; Mikysek, T.; Švancara, I.; Metelka, R. Simultaneous Determination of Lipophilic Vitamin Esters Using Square-wave Voltammetry at the Glassy Carbon Electrode. Electroanalysis 2021, 33, 537–542. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. Glassy Carbon Electrodes Film-Modified with Acidic Functionalities. A Review. Electroanalysis 2012, 24, 1481–1500. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Adamczyk, M. New Strategies for the Simple and Sensitive Voltammetric Direct Quantification of Se(IV) in Environmental Waters Employing Bismuth Film Modified Glassy Carbon Electrode and Amberlite Resin. Molecules 2021, 26, 4130. [Google Scholar] [CrossRef]
- Kulpa-Koterwa, A.; Ossowski, T.; Niedziałkowski, P. Functionalized Fe3O4 Nanoparticles as Glassy Carbon Electrode Modifiers for Heavy Metal Ions Detection—A Mini Review. Materials 2021, 14, 7725. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene based sensors and biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Smarzewska, S.; Miękoś, E.; Guziejewski, D.; Zieliński, M.; Burnat, B. Graphene oxide activation with a constant magnetic field. Anal. Chim. Acta 2018, 1011, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Sattar, T. Current Review on Synthesis, Composites and Multifunctional Properties of Graphene. Top. Curr. Chem. 2019, 377, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Qing, M.Q.; Wang, Y.B.; Chen, S.M. Defects in Graphene: Generation, Healing, and Their Effects on the Properties of Graphene: A Review. J. Mater. Sci. Technol. 2015, 31, 599–606. [Google Scholar] [CrossRef]
- Kumar, P.; Wani, M.F. Synthesis and tribological properties of graphene: A review. J. Tribol. 2017, 13, 36–71. [Google Scholar]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Beitollahi, H.; Hamzavi, M.; Torkzadeh-Mahani, M. Electrochemical determination of hydrochlorothiazide and folic acid in real samples using a modified graphene oxide sheet paste electrode. Mater. Sci. Eng. C 2015, 52, 297–305. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.; Banks, C.E. Graphene Oxide Bulk-Modified Screen-Printed Electrodes Provide Beneficial Electroanalytical Sensing Capabilities. Biosensors 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Isin, D.; Eksin, E.; Erdem, A. Graphene oxide modified single-use electrodes and their application for voltammetric miRNA analysis. Mater. Sci. Eng. C 2017, 75, 1242–1249. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Devadas, B.; Chen, S.M. Highly sensitive and selective determination of pyrazinamide at poly-L-methionine/reduced graphene oxide modified electrode by differential pulse voltammetry in human blood plasma and urine samples. J. Colloid Interface Sci. 2014, 418, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Shen, T.Z.; Song, J.K. Water front recession and the formation of various types of wrinkles in dried graphene oxide droplets. Carbon 2016, 105, 297–304. [Google Scholar] [CrossRef]
- Wang, L.M.; Dong, J.B.; Wang, Y.L.; Cheng, Q.; Yang, M.M.; Cai, J.; Liu, F.Q. Novel Signal-Amplified Fenitrothion Electrochemical Assay, Based on Glassy Carbon Electrode Modified with Dispersed Graphene Oxide. Sci. Rep. 2016, 6, 23409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anson, F.C. Application of Potentiostatic Current Integration to the Study of the Adsorption of Cobalt(III)-(Ethylenedinitrilo(tetraacetate) on Mercury Electrodes. Anal. Chem. 1964, 36, 932–934. [Google Scholar] [CrossRef]
- Christie, J.H.; Lauer, G.; Osteryoung, R.A.; Anson, F.C. Determination of Charge Passed Following Application of Potential Step in Study of Electrode Processes. Anal. Chem. 1963, 35, 1979. [Google Scholar] [CrossRef]
- Yu, L.L.; Zheng, H.J.; Shi, M.X.; Jing, S.S.; Qu, L.B. A Novel Electrochemical Sensor Based on Poly (Diallyldimethylammonium Chloride)-Dispersed Graphene Supported Palladium Nanoparticles for Simultaneous Determination of Sunset Yellow and Tartrazine in Soft Drinks. Food Anal. Method 2017, 10, 200–209. [Google Scholar] [CrossRef]
- Gosser, D. Cyclic Voltammetry; Simulation and Analysis of Reaction Mechanisms. Synth. React. Inorg. Met.-Org. Chem. 1994, 24, 1237–1238. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Ciesielski, W.; Sokolowski, A. Theoretical and experimental study of the catalytic hydrogen evolution reaction in the presence of an adsorbed catalyst by means of square-wave voltammetry. J. Electroanal. Chem. 2005, 585, 97–104. [Google Scholar] [CrossRef]
- Mirceski, V.; Gulaboski, R. Surface catalytic mechanism in square-wave voltammetry. Electroanalysis 2001, 13, 1326–1334. [Google Scholar] [CrossRef]
- Mirceski, V.; Gulaboski, R. The surface catalytic mechanism: A comparative study with square-wave and staircase cyclic voltammetry. J. Solid State Electrochem. 2003, 7, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Skrzypek, S.; Mirceski, V.; Smarzewska, S.; Guziejewski, D.; Ciesielski, W. Voltammetric Study of 2-Guanidinobenzimidazole: Electrode Mechanism and Determination at Mercury Electrode. Collect. Czech. Chem. Commun. 2011, 76, 1699–1715. [Google Scholar] [CrossRef]
- Goncalves, L.M.; Batchelor-McAuley, C.; Barros, A.A.; Compton, R.G. Electrochemical Oxidation of Adenine: A Mixed Adsorption and Diffusion Response on an Edge-Plane Pyrolytic Graphite Electrode. J. Phys. Chem. C 2010, 114, 14213–14219. [Google Scholar] [CrossRef]
- Erk, N. Differential pulse anodic voltammetric determination of pantoprazole in pharmaceutical dosage forms and human plasma using glassy carbon electrode. Anal. Biochem. 2003, 323, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Brett, C.; Oliveira Brett, A.M. Electroanalysis; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Compton, R.G.; Laborda, E.; Ward, K.R. Understanding Voltammetry: Simulation of Electrode Process; Imperial College Press: London, UK, 2011. [Google Scholar]
- Zhu, X.H.; Zeng, L.X.; Xu, M.T.; Liang, Y.; Nan, J.M. A glassy carbon electrode modified with electrochemically reduced graphene for simultaneous determination of guanine and adenine. Anal. Methods 2012, 4, 2935–2939. [Google Scholar] [CrossRef]
- Dryhurst, G.; Elving, P.J. Electrochemical Oxidation of Adenine—Reaction Products and Mechanisms. J. Electrochem. Soc. 1968, 115, 1014. [Google Scholar] [CrossRef]
- Niedzialkowski, P.; Bogdanowicz, R.; Zieba, P.; Wysocka, J.; Ryl, J.; Sobaszek, M.; Ossowski, T. Melamine-modified Boron-doped Diamond towards Enhanced Detection of Adenine, Guanine and Caffeine. Electroanalysis 2016, 28, 211–221. [Google Scholar] [CrossRef]
- Milazzo, G. (Ed.) Topics in Bioelectrochemistry and Bioenergetics; John Wiley and Sons: London, UK, 1983; Volume V. [Google Scholar]
- Oliveira-Brett, A.M.; Diculescu, V.; Piedade, J.A.P. Electrochemical oxidation mechanism of guanine and adenine using a glassy carbon microelectrode. Bioelectrochemistry 2002, 55, 61–62. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, S.A.; Kauffmann, J.M.; Zuman, P. Electroanalytical Method Validation in Pharmaceutical Analysis and Their Applications. In Electroanalysis in Biomedical and Pharmaceutical Sciences; Monographs in Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 235–266. [Google Scholar] [CrossRef]










| Hg(Ag)FE | GO/GC | |
|---|---|---|
| Linear range (mol L–1) | 5.0 × 10–7–7.0 × 10–6 | 3.0 × 10–7–3.0 × 10–5 |
| LOD (mol L–1) | 1.35 × 10−7 | 4.86 × 10−8 |
| LOQ (mol L–1) | 4.48 × 10−7 | 1.62 × 10−7 |
| R2 | 0.9999 | 0.9992 |
| Reproducibility of the peak current, CV (%) | 6.6 | 6.7 |
| Reproducibility of the peak potential, CV (%) | 2.5 | 4.3 |
| Repeatability of the procedure, CV (%) | 3.9 | 3.8 |
| Method | Surface Modification If Applicable and Electrode Type | Sample Type | Linear Concentration Range [mol L–1] | LOD [mol L–1] | LOQ [mol L–1] | Reference |
|---|---|---|---|---|---|---|
| adsorptive stripping differential pulse and square-wave voltammetry | bare GCE | pharmaceutical formulation | 6.0 × 10−7–6.0 × 10−5 both | 1.02 × 10−7 and 8.40 × 10−8, respectively | 3.39 × 10−7 and 2.80 × 10−7, respectively | [14] |
| adsorptive stripping square wave voltammetry | benzalkonium chloride coated AgNP on GCE | pharmaceutical dosage form | 6.0 × 10−8–1.0 × 10−6 | 2.39 × 10−9 | 7.96×10−9 | [13] |
| cathodic adsorptive stripping square-wave voltammetry | hanging mercury drop electrode | human plasma and tablet formulation | 1.7 × 10−6–1.7 × 10−5 | - | 1.3×10−6 | [11] |
| differential pulse voltammetry | nickel-cobalt sulphide decorated graphene quantum dots on GCE | pharmaceutical formulation, spiked urine | 5.0 × 10−6–1.8 × 10−5 | 1.21 × 10−8 | - | [10] |
| square wave voltammetry | boron-doped diamond electrode | pharmaceutical formulation | 5.0 × 10−6–1.0 × 10−4 | 1.9 × 10−6 | 5.6 × 10−7 | [12] |
| square wave voltammetry | renewable silver amalgam electrode | pharmaceutical formulations | 5.0 × 10–7–7.0 × 10–6 | 1.35 × 10−7 | 4.48 × 10−7 | This work |
| square wave voltammetry | graphene oxide on GCE | spiked and real patients urine | 3.0 × 10–7–3.0 × 10–5 | 4.86 × 10−8 | 1.62 × 10−7 | This work |
| Pharmaceutical Formulation | Declared (mg) | Found (mg) | Recovery (%) |
|---|---|---|---|
| Viread | 245.0 | 238.8 ± 14.1 | 97.5 |
| Tenofovir disoproxil Teva | 245.0 | 255.8 ± 14.3 | 104.4 |
| Declared (μmol·L−1) | Found (μmol·L−1) | CV (%) | Recovery (%) |
|---|---|---|---|
| 10.0 | 10.3 ± 0.9 | 9.5 | 103.0 |
| 50.0 | 47.7 ± 4.0 | 9.7 | 95.5 |
| 100.0 | 97.5 ± 7.6 | 8.9 | 97.5 |
| Patient | TFV Content µg × mL−1 | |
|---|---|---|
| SWV | HPLC | |
| I | 98.4 ± 8.1 | 86.8 ± 7.8 |
| II | 88.7 ± 7.5 | 80.1 ± 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festinger, N.; Spilarewicz-Stanek, K.; Borowczyk, K.; Guziejewski, D.; Smarzewska, S. Highly Sensitive Determination of Tenofovir in Pharmaceutical Formulations and Patients Urine—Comparative Electroanalytical Studies Using Different Sensing Methods. Molecules 2022, 27, 1992. https://doi.org/10.3390/molecules27061992
Festinger N, Spilarewicz-Stanek K, Borowczyk K, Guziejewski D, Smarzewska S. Highly Sensitive Determination of Tenofovir in Pharmaceutical Formulations and Patients Urine—Comparative Electroanalytical Studies Using Different Sensing Methods. Molecules. 2022; 27(6):1992. https://doi.org/10.3390/molecules27061992
Chicago/Turabian StyleFestinger, Natalia, Kaja Spilarewicz-Stanek, Kamila Borowczyk, Dariusz Guziejewski, and Sylwia Smarzewska. 2022. "Highly Sensitive Determination of Tenofovir in Pharmaceutical Formulations and Patients Urine—Comparative Electroanalytical Studies Using Different Sensing Methods" Molecules 27, no. 6: 1992. https://doi.org/10.3390/molecules27061992