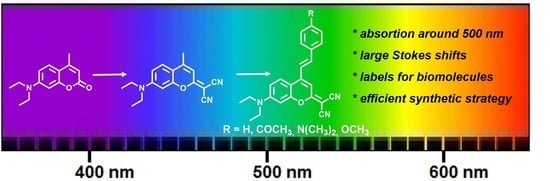
New Red-Shifted 4-Styrylcoumarin Derivatives as Potential Fluorescent Labels for Biomolecules
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Photophysical Properties
2.3. Theoretical Calculations
3. Materials and Methods
3.1. General Methods
3.2. Procedures for the Preparation of Compounds
3.2.1. 2-(7-(Diethylamino)-4-methyl-2H-chromen-2-ylidene)malononitrile (3)
3.2.2. (E)-Methyl 4-(2-(2-(dicyanomethylene)-7-(diethylamino)-2H-chromen-4-yl)benzoate (4)
3.2.3. (E)-2-(7-(Diethylamino)-4-styryl)-2H-chromen-2-ylidene)malononitrile (5)
3.2.4. (E)-2-(7-(Diethylamino)-4-(4-(dimethylamine)Styryl)-2H-chromen-2-ylidene)malononitrile (6)
3.2.5. (E)-2-(7-(Diethylamino)-4-(4-methoxystyryl)-2H-chromen-2-ylidene)malononitrile (7)
3.2.6. (E)-6-(4-(2-(2-(Dicyanomethylene)-7-(diethylamino)-2H-chromen-4-yl)vinyl)phenoxy)hexanoic Acid (8)
3.2.7. (E)-2,5-Dioxopyrrolidin-1-yl 6-(4-(2-(2-(dicyanomethylene)-7-(diethylamino)-2H-chromen-4-Yl)vinyl)phenoxy)hexanoate (9)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Modesti, M. Fluorescent Labeling of Proteins. Methods Mol. Biol. 2018, 1665, 115–134. [Google Scholar] [PubMed]
- Nath, N.; Becky Godat, B.; Urh, M. Antibody Labeling with Fluorescent Dyes Using Magnetic Protein A and Protein G Beads. J. Vis Exp. 2016, 115, e54545. [Google Scholar] [CrossRef]
- Dirks, R.W.; Tanke, H.J. Advances in fluorescent tracking of nucleic acids in living cell. Biotechniques 2006, 40, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogera, O.; Colliec-Jouaulta, S.; Ratiskola, J.; Sinquina, C.; Guezenneca, J.; Fischerb, A.M.; Chevolota, L. Polysaccharide labelling: Impact on structural and biological properties. Carbohyd. Polym. 2002, 50, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Fairn, G.D. Molecular probes to visualize the location, organization and dynamics of lipids. J. Cell Sci. 2014, 127, 4801–4812. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Martins, S.; Caldeira, A.T. Coumarins as Fluorescent Labels of Biomolecules. In Phytochemicals in Human Health; Rao, V., Ed.; IntechOpen Publications: London, UK, 2019; pp. 1–19. [Google Scholar]
- Giepmans, B.; Adams, S.; Ellisman, M.; Tsien, R. The fluorescent toolbox for assessing protein location and function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, M. Green fluorescent protein (GFP): Applications, structure, and related photophysical behavior. Chem. Rev. 2002, 102, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Staveren, D.; Metzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef]
- Soh, N.; Yoshida, K.; Nakajima, H.; Nakano, K.; Imato, T.; Fukaminato, T.; Irie, M. A fluorescent photochromic compound for labeling biomolecules. Chem. Commun. 2007, 48, 5206–5208. [Google Scholar] [CrossRef]
- Benedetto, F.; Mele, E.; Camposeo, A.; Athanassiou, A.; Cingolani, R.; Pisignano, D. Photoswitchable Organic Nanofibers. Adv. Mater. 2008, 20, 314–318. [Google Scholar] [CrossRef]
- Li, H.; Rothberg, L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 14036–14039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A. Radioactive tracer techniques in the sequencing of glycoprotein oligosaccharides. FASEB J. 1991, 5, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Mann, M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC). Nat. Protoc. 2006, 1, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H. Fluorescent labeling techniques in biomolecules: A flashback. RSC Adv. 2012, 2, 7017–7029. [Google Scholar] [CrossRef]
- Hanson, G.; Hanson, B. Fluorescent probes for cellular assays. Comb. Chem. High Throughput Screen. 2008, 11, 505–513. [Google Scholar] [CrossRef]
- Maurel, D.; Comps-Agrar, L.; Brock, C.; Rives, M.; Bourrier, E.; Ayoub, M.; Bazin, H.; Tinel, N.; Durroux, T.; Prézeau, L.; et al. Cell-surface protein–protein interaction analysis with time-resolved FRET and snap-tag technologies: Application toGPCR oligomerization. Nat. Methods 2008, 5, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Straight, P.; McLoughlin, S.; Zhou, Z.; Lin, A.; Golan, D.; Kelleher, N.; Kolter, R.; Walsh, C. Genetically encoded short peptide tag for versatile protein labeling by Sfp phosphopantetheinyl transferase. Proc. Natl. Acad. Sci. USA 2005, 102, 15815–15820. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, T. Bioconjugate Techniques, 3rd ed.; Elsevier: London, UK, 2013. [Google Scholar]
- Fang, X.; Zheng, Y.; Duan, Y.; Liu, Y.; Zhong, W. Recent Advances in Design of Fluorescence-Based Assays for High-Throughput Screening. Anal. Chem. 2019, 91, 482–504. [Google Scholar] [CrossRef]
- Johnson, I.; Spence, M. Molecular Probes™ Handbook—A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies; Thermo Fischer Scientific: Waltham, MA, USA, 2010. [Google Scholar]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2009, 110, 2620–2640. [Google Scholar] [CrossRef] [Green Version]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Riveiro, M.; De Kimpe, N.; Moglioni, A.; Vázquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yen, C.; Yang, W.; Chen, H.; Liao, C.; Tsai, C.; Chen, C. Efficient green coumarin dopants for organic light-emitting devices. Org. Lett. 2004, 6, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Avó, J.; Lima, J.; Nogueira, J.; Andrade, L.; Mendes, A.; Pereira, A.; Branco, P.S. Styryl and phenylethynyl based coumarin chromophores for dye sensitized solar cells. J. Photochem. Photobiol. A Chem. 2018, 353, 564–569. [Google Scholar] [CrossRef]
- Lanke, S.K.; Sekar, N. Coumarin Push-Pull NLOphores with red emission: Solvatochromic and theoretical approach. J. Fluoresc. 2016, 26, 949–962. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Guo, M.; Du, L.; Liu, G.; Wang, P. Synthesis of polymeric fluorescent brightener based on coumarin and its performances on paper as light stabilizer, fluorescent brightener and surface sizing agent. Appl. Surf. Sci. 2016, 367, 167–173. [Google Scholar] [CrossRef]
- Wilze, K.; Johnson, A. Handbook of Detergents, Chemistry, Production, and Application of Fluorescent Whitening Agents, Part, F; Taylor & Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zaorska, E.; Konop, M.; Ostaszewski, R.; Koszelewski, D.; Ufnal, M. Salivary Hydrogen Sulfide Measured with a New Highly Sensitive Self-Immolative Coumarin-Based Fluorescent Probe. Molecules 2018, 23, 2241. [Google Scholar] [CrossRef] [Green Version]
- Gandioso, A.; Bresolí-Obach, R.; Nin-Hill, A.; Bosch, M.; Palau, M.; Galindo, A.; Contreras, S.; Rovira, A.; Rovira, C.; Nonell, S.; et al. Redesigning the coumarin scaffold into small bright fluorophores with far-red to NIR emission and large Stokes’ shifts useful for cell imaging. J. Org. Chem. 2018, 83, 1185–1195. [Google Scholar] [CrossRef]
- Bojtár, M.; Kormos, A.; Kis-Petik, K.; Kellermayer, M.; Kele, P. Green-Light Activatable, Water-Soluble Red-Shifted Couma-rin Photocages. Org. Lett. 2019, 21, 9410–9414. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.; Branco, P.; Pereira, A. An Efficient Methodology for the Synthesis of 3-Styryl Coumarins. J. Braz. Chem. Soc. 2012, 23, 688–693. [Google Scholar] [CrossRef]
- Gordo, J.; Avó, J.; Parola, J.; Lima, J.; Pereira, A.; Branco, P. Convenient Synthesis of 3-Vinyl and 3-Styryl Coumarins. Org. Lett. 2011, 13, 5112–5115. [Google Scholar] [CrossRef]
- Gandioso, A.; Contreras, S.; Melnyk, I.; Oliva, J.; Nonell, S.; Velasco, D.; Garcia-Amorós, J.; Marchán, V. Development of green/red-absorbing chromophores based on a coumarin scaffold useful as caging groups. J. Org. Chem. 2017, 82, 5398–5408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, J.; Won, M.; Park, S.; Park, K.; Shin, D.-S.; Kim, J.; Lee, M. A dicyanocoumarin-fused quinolinium based probe for NAD(P)H and its use for detecting glycolysis and hypoxia in living cells and tumor spheroids. Sens. Actuators B Chem. 2020, 320, 128360. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Finney, N.S. Small-molecule fluorescent probes and their design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [Green Version]
- Sednev, M.V.; Belov, V.N.; Hell, S.W. Fluorescent dyes with large Stokes shifts for super-resolution optical microscopy of biological objects: A review. Methods Appl. Fluoresc. 2015, 3, 042004. [Google Scholar] [CrossRef] [Green Version]
- Cisse, L.; Djande, A.; Capo-Chichi, M.; Khonté, A.; Bakhoum, J.; Delattre, F.; Yoda, J.; Saba, A.; Tine, A.; Aaron, J. Quantitative study of the substituent effects on the electronic absorption and fluorescence spectra of coumarins. J. Phys. Org. Chem. 2019, 33, e4014. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Guo, H.; Xie, L. Geometry Relaxation-Induced Large Stokes Shift in Red-Emitting Borondipyrromethenes (BODIPY) and Applications in Fluorescent Thiol Probes. J. Org. Chem. 2012, 77, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Y.; Zhang, H.; He, X. Near infrared absorption/emission perylenebisimide fluorophores with geometry relaxation-induced large Stokes shift. RSC Adv. 2020, 10, 35840–35847. [Google Scholar] [CrossRef]
- Jaggi, M.; Blum, C.; Marti, B.S.; Liu, S.X.; Leutwyler, S.; Decurtins, S. Annulation of Tetrathiafulvalene to the Bay Region of Perylenediimide. Org. Lett. 2010, 12, 1344–1347. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Wang, J.; Durbeej, B. How accurate are TD-DFT excited-state geometries compared to DFT ground-state geometries? J. Comput. Chem. 2020, 41, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Amovilli, C.; Barone, V.; Cammi, R.; Cancès, E.; Cossi, M.; Mennucci, B.; Pomelli, C.S.; Tomasi, J. Recent Advances in the Description of Solvent Effects with the Polarizable Continuum Model. J. Adv. Quantum Chem. 1998, 32, 227–261. [Google Scholar]
- Cossi, M.; Barone, V. Time-dependent density functional theory for molecules in liquid solutions. J. Chem. Phys. 2001, 115, 4708–4717. [Google Scholar] [CrossRef]





| Compound | λabs a (nm) | λem b (nm) | Stokes Shift (nm, cm−1) | ε c (cm−1M−1) | ΦF d |
|---|---|---|---|---|---|
| 1 | 371 | 434 | 63, 3913 | 22,910 | 0.73 |
| 3 | 477 | 519 | 42, 1697 | 38,000 | 0.05 |
| 4 | 520 | 620 | 100, 3102 | 19,000 | 0.04 |
| 5 | 516 | 603 | 87, 2796 | 17,000 | 0.16 |
| 6 | 496 | 597 | 101, 3411 | 34,000 | 0.95 |
| 7 | 522 | 602 | 80, 2546 | 24,000 | 0.20 |
| 8 | 519 | 596 | 77, 2489 | 24,000 | 0.28 |
| 9 | 523 | 597 | 74, 2370 | 24,000 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eustáquio, R.; Ramalho, J.P.P.; Caldeira, A.T.; Pereira, A. New Red-Shifted 4-Styrylcoumarin Derivatives as Potential Fluorescent Labels for Biomolecules. Molecules 2022, 27, 1461. https://doi.org/10.3390/molecules27051461
Eustáquio R, Ramalho JPP, Caldeira AT, Pereira A. New Red-Shifted 4-Styrylcoumarin Derivatives as Potential Fluorescent Labels for Biomolecules. Molecules. 2022; 27(5):1461. https://doi.org/10.3390/molecules27051461
Chicago/Turabian StyleEustáquio, Raquel, João P. Prates Ramalho, Ana T. Caldeira, and António Pereira. 2022. "New Red-Shifted 4-Styrylcoumarin Derivatives as Potential Fluorescent Labels for Biomolecules" Molecules 27, no. 5: 1461. https://doi.org/10.3390/molecules27051461