pH as a Key Factor for the Quality Assurance of the Preparation of Gastrodiae Rhizoma Formula Granules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantitative Analysis of Index Components in GR by HPLC
Determination of Six Index Components in GR Real Samples
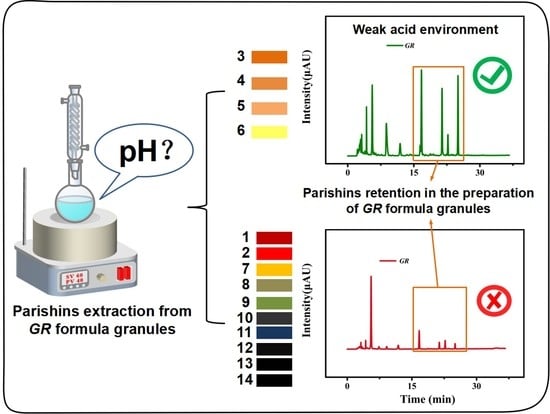
2.2. The Effect of pH on the Transfer Rate of Parishins in GR Formula Granules
2.3. Importance of pH in the Preparation of GR Formula Granules
3. Materials and Methods
3.1. Materials, Reagents, and Chemicals
3.2. Instruments
3.3. HPLC Analysis
3.3.1. Preparation of Standard and Sample Solutions
3.3.2. HPLC Analysis
3.4. Calculation of the Transfer Rate of Paste Extraction
3.5. Investigation on Hydrolysis Conditions of Parishin A
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The National Pharmacopoeia Commission of P.R. China. Pharmacopoeia of the People’s Republic of China; China Medicine Science Technology Press: Beijing, China, 2015; pp. 54–55. [Google Scholar]
- Hu, M.; Yan, H.; Fu, Y.; Jiang, Y.; Yao, W.; Yu, S.; Zhang, L.; Wu, Q.; Ding, A.; Shan, M. Optimal Extraction Study of Gastrodin-Type Components from Gastrodia elata Tubers by Response Surface Design with Integrated Phytochemical and Bioactivity Evaluation. Molecules 2019, 24, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, Y.; Zhang, Z.; Hu, Y.; Cui, X.; Xiong, Y. Quality Evaluation of Gastrodia elata Tubers Based on HPLC Fingerprint Analyses and Quantitative Analysis of Multi-Components by Single Marker. Molecules 2019, 24, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, E.; Hong, Y.Y.; Chen, Y.; Tao, L.; Xu, Y.; Chen, T.; Shen, X. Gastrodin Ameliorates Cognitive Dysfunction in Vascular Dementia Rats by Suppressing Ferroptosis via the Regulation of the Nrf2/Keap1-GPx4 Signaling Pathway. Molecules 2022, 27, 6311. [Google Scholar] [CrossRef]
- Matias, M.; Silvestre, S.; Falcão, A.; Alves, G. Gastrodia elata and epilepsy, Rationale and therapeutic potential. Pharmacol. Res. 2016, 23, 1511–1526. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Wang, D.; Yang, S.; Feng, Y. Myocardial protection properties of parishins from the roots of Gastrodia elata Bl. Biomed. Pharmacother. 2019, 121, 109645. [Google Scholar] [CrossRef]
- Chen, C.; Fu, Y.; Li, M.; Ruan, L.; Xu, H.; Chen, J.; Zhao, W.; Meng, H.; Xing, Y.; Hong, W.; et al. Nuclear magnetic resonance-based metabolomics approach to evaluate preventive and therapeutic effects of Gastrodia elata Blume on chronic atrophic gastritis. J. Pharm. Biomed. Anal. 2019, 164, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Liu, D.; Mu, Y.; Dong, H.; Zhou, H.; Wang, X. Chemical constituents from the rhizomes of Gastrodia elata f. glauca and their potential neuroprotective effects. Phytochem. Lett. 2018, 24, 167–171. [Google Scholar] [CrossRef]
- YBZ-PFKL-2021122; National Medical Products Administration, National Drug Standard. Standards Press of China: Beijing, China, 2021.
- Liu, Y.; Gao, J.; Peng, M.; Meng, H.; Ma, H.; Cai, P.; Xu, Y.; Zhao, Q.; Si, G. A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Huang, L.; Shi, L.; Zheng, Z.; Chen, J.; Qu, Y.; Xiao, H.; Luo, H.; Wu, G. Para-Hydroxybenzyl Alcohol Delays the Progression of Neurodegenerative Diseases in Models of Caenorhabditis elegans through Activating Multiple Cellular Protective Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 8986287. [Google Scholar] [CrossRef]
- Kaengkan, P.; Baek, S.; Choi, Y.; Kam, K.; Kim, J.; Wu, Y.; Do, B.; Kang, S. Combination effect of p-hydroxybenzyl alcohol and mesenchymal stem cells on the recovery of brain damage in a rat model of brain ischemia. Anim. Cells Syst. 2013, 17, 160–169. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, S.; Yan, R.; Gong, C.; Gui, Q.; Zhang, Q.; Xiang, L.; Chen, L.; Wang, P.; Li, S.; et al. Parishin from Gastrodia elata Ameliorates Aging Phenotype in Mice in a Gut Microbiota-Related Manner. Front. Microbiol. 2022, 13, 877099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Feng, N.; Wang, L.; Shi, J.; Wang, X. Parishin C’s prevention of A β 1-42-induced inhibition of long-term potentiation is related to NMDA receptors. Acta Pharm. Sin. B 2016, 3, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Sun, Y.; Weng, Y.; Matsuura, A.; Xiang, L.; Qi, J. Parishin from Gastrodia elata Extends the Lifespan of Yeast via Regulation of Sir2/Uth1/TOR Signaling Pathway. Oxidative Med. Cell. Longev. 2016, 2016, 4074690. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Han, Y.; Gui, L.; Sun, J.; Chen, Y.; Song, R.; Guo, J.; Xie, Y.; Lu, D.; Sun, L. Gastrodin attenuation of the inflammatory response in H9c2 cardiomyocytes involves inhibition of NF-kB and MAPKs activation via the phosphatidylinositol 3-kinase signaling. Biochem. Pharmacol. 2013, 85, 1124–1133. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Liu, X.; Cheng, M.; Qu, Y.; Xiao, H. Comparative pharmaco-kinetics of gastrodin in rats after intragastric administration of free gastrodin, parishin and Gastrodia elata extract. J. Ethnopharmacol. 2015, 176, 49–54. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ouyang, H.; Lu, Y.; Qiu, Y.; Feng, Y.; Jiang, H.; Zhou, X.; Yang, S. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodia elata using UHPLC/Q-TOF-MS/MS. J. Chromatogr. B 2015, 988, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Y.; Zhao, J.; Wang, M.; Avula, B.; Peng, Q.; Ouyang, H.; Zhong, L.; Zhang, J.; Khan, I. Identification and Characterization of Key Chemical Constituents in Processed Gastrodia elata Using UHPLC-MS/MS and Chemometric Methods. J. Anal. Methods Chem. 2019, 2019, 4396201. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Wang, L.; Li, J.; Liu, X.; Cheng, M.; Xiao, H. Analysis of the metabolic profile of parishin by ultra-performance liquid chromatography/quadrupole-time of flight mass spectrometry. Biomed. Chromatogr. 2015, 29, 1913–1920. [Google Scholar] [CrossRef]
- Wong, H.; Hu, B.; So, P.; Chan, C.; Mok, D.; Xin, G.; Li, P.; Yao, Z. Rapid authentication of Gastrodiae rhizoma by direct ionization mass spectrometry. Anal. Chim. Acta 2016, 938, 90–97. [Google Scholar] [CrossRef]
- Liu, Y.; Yi, J.; Chen, Y.; Liu, Z.; Liu, Y. RP-HPLC detemination of free gastrodin and total gastrodin in Gastrodia elata. Chin. J. Pharm. Anal. 2010, 30, 30–32. [Google Scholar] [CrossRef]
- Yang, R.; Min, K.; Wang, Y.; Chen, S.; Ma, M.; Li, M.; Yan, J.; Chen, B.; Yao, S. Rapid semi-quantitative analysis of hemolytic triterpenoid saponins in Lonicerae Flos crude drugs and preparations by paper spray mass spectrometry. Talanta 2022, 239, 123148. [Google Scholar] [CrossRef] [PubMed]
- Meston, D.; Themelis, T.; Zhou, Z.; De Vos, J.; De Pra, M.; Steiner, F.; Becue, I.; Daeseleire, E.; Desmet, G.; Eeltink, S. Development of a generic ultra-high-pressure gradient liquid-chromatography method development protocol: The analysis of residual multi-class antibiotics in food products as a case study. J. Chromatogr. A 2022, 1684, 463565. [Google Scholar] [CrossRef]
- Avila, L.M.; dos Santosb, A.F.; de Mattosb, D.M.; de Souza, C.G.; de Andrade, D.F.; d’Avila, L.A. Determination of ethanol in gasoline by high-performance liquid chromatography. Fuel 2018, 212, 236–239. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Yang, P.; Song, P. Present situation and new development ideas of traditional Chinese medicine granules. Zhong Cao Yao 2018, 49, 4717–4725. [Google Scholar] [CrossRef]
- Yang, L.; Jun, H.; Geng, L.; Tan, J.; Wang, L.; Qian, Z.; Zhang, W.; Song, Z. Discussion on the overall quality control mode of traditional Chinese medicine based on standard decoction. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 1–6. [Google Scholar] [CrossRef]
- Xie, T. General situation of research on equivalence between traditional Chinese medicine granules and standard decoction. Guangdong Chem. Ind. 2021, 48, 142–143. [Google Scholar] [CrossRef]
- Ni, L.; Dai, Y.; Dou, Z.; Wang, Z.; Zhou, Y. Study on law of quality value transmitting of Artemisiae Scopariae Herba standard decoction. Zhong Cao Yao 2020, 51, 2954–2966. [Google Scholar] [CrossRef]
- Ling, L.; Lei, C.; Liu, H.; Zhang, J.; Xiang, A.; Lin, S.; Zhu, J.; Huang, D. Preparation and Quality Evaluation Study on Gastrodia elata Standard Decoction. Asia Pac. Tradit. Med. 2020, 16, 43–47. [Google Scholar] [CrossRef]
- Tian, Z.; Xiao, H.; Feng, S.; Liu, D.; Cheng, J.; Chen, S. Analysis of degradation law of parishins in effective components of Gastrodia elata Bge. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 18–21. [Google Scholar] [CrossRef]






| Compound | Calibration Curve | Uncertainties of Slopes | Uncertainties of Intercepts | Linear Range (mg/L) | R2 | LOD (mg/L) | LOQ (mg/L) | T |
|---|---|---|---|---|---|---|---|---|
| gastrodin | y = 20,322 x − 47,498 | 50 | 2.6 × 104 | 20–1000 | 0.9999 | 0.040 | 0.130 | 0.928 |
| p-hydroxybenzyl alcohol | y = 37,946 x − 30,919 | 136 | 6.8 × 104 | 5–1000 | 0.9996 | 0.035 | 0.090 | 1.045 |
| parishin A | y = 18,330 x − 36,509 | 98 | 5.7 × 104 | 20–1200 | 0.9999 | 0.050 | 0.150 | 1.028 |
| parishin B | y = 16,156 x − 4942.5 | 100 | 5.7 × 104 | 20–1200 | 0.9999 | 0.050 | 0.150 | 1.039 |
| parishin C | y = 13,934 x − 1890.9 | 96 | 5.5 × 104 | 1–1000 | 0.9996 | 0.057 | 0.185 | 1.001 |
| parishin E | y = 12,300 x − 12,5776 | 96 | 5.6 × 104 | 20–1200 | 0.9997 | 0.500 | 1.650 | 0.848 |
| Samples | No. | Mass Fraction (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| Gastrodin | p-Hydroxybenzyl Alcohol | Parishin A | Parishin B | Parishin C | Parishin E | ||
| GR decoction pieces | S1 | 4.1 ± 1.9 | 0.2 ± 1.9 | 7.8 ± 0.6 | 3.7 ± 0.7 | 1.7 ± 0.7 | 6.3 ± 0.8 |
| S2 | 1.6 ± 1.8 | 0.1 ± 1.1 | 3.6 ± 2.8 | 2.0 ± 1.6 | 0.8 ± 2.8 | 4.3 ± 2.1 | |
| S3 | 5.0 ± 2.2 | 1.5 ± 0.7 | 8.8 ± 0.5 | 7.3 ± 0.6 | 2.6 ± 0.6 | 9.6 ± 1.6 | |
| S4 | 4.2 ± 2.2 | 0.3 ± 1.6 | 6.3 ± 1.8 | 4.8 ± 2.5 | 1.7 ± 1.7 | 12.6 ± 2.2 | |
| S5 | 2.9 ± 2.2 | 0.2 ± 2.1 | 4.0 ± 1.3 | 3.6 ± 1.2 | 1.1 ± 2.1 | 9.5 ± 0.8 | |
| S6 | 1.6 ± 1.4 | 0.2 ± 1.4 | 4.1 ± 1.1 | 2.6 ± 1.7 | 0.6 ± 2.3 | 8.3 ± 2.1 | |
| S7 | 1.4 ± 2.1 | 0.8 ± 0.9 | 6.0 ± 2.3 | 3.2 ± 2.1 | 0.8 ± 1.8 | 8.4 ± 1.6 | |
| S8 | 3.3 ± 0.2 | 0.6 ± 2.0 | 10.2 ± 1.7 | 5.1 ± 0.6 | 1.3 ± 2.1 | 6.0 ± 2.3 | |
| S9 | 1.8 ± 1.1 | 0.5 ± 1.1 | 1.4 ± 1.8 | 1.8 ± 2.0 | 0.4 ± 1.1 | 6.7 ± 1.7 | |
| S10 | 2.3 ± 1.0 | 0.5 ± 1.7 | 3.8 ± 1.5 | 2.9 ± 0.8 | 0.8 ± 0.3 | 8.7 ± 0.7 | |
| S11 | 2.5 ± 1.9 | 0.5 ± 0.5 | 6.1 ± 2.1 | 4.0 ± 1.4 | 1.2 ± 1.9 | 4.9 ± 2.6 | |
| S12 | 1.6 ± 2.0 | 1.8 ± 0.7 | 3.4 ± 2.4 | 4.0 ± 0.9 | 0.8 ± 1.0 | 10.0 ± 0.7 | |
| S13 | 1.6 ± 1.2 | 1.8 ± 1.2 | 4.0 ± 1.1 | 4.6 ± 1.2 | 0.8 ± 1.5 | 8.0 ± 1.0 | |
| S14 | 1.5 ± 1.8 | 0.4 ± 1.5 | 3.3 ± 1.7 | 1.9 ± 0.8 | 0.5 ± 1.8 | 4.7 ± 4.1 | |
| GR standard decoction | Y1 | 20.2 ± 0.7 | 2.8 ± 3.4 | 20.9 ± 0.4 | 12.8 ± 0.3 | 5.3 ± 0.7 | 15.5 ± 0.9 |
| Y2 | 11.1 ± 0.8 | 3.4 ± 2.0 | 10.4 ± 2.6 | 9.9 ± 1.5 | 2.9 ± 2.8 | 13.5 ± 3.1 | |
| Y3 | 24.4 ± 1.2 | 12.7 ± 2.0 | 20.9 ± 2.3 | 21.8 ± 0.5 | 8.3 ± 1.6 | 19.5 ± 3.5 | |
| Y4 | 11.1 ± 3.7 | 2.2 ± 3.1 | 6.1 ± 3.2 | 8.6 ± 3.8 | 2.5 ± 3.2 | 23.6 ± 3.7 | |
| Y5 | 17.1 ± 2.9 | 1.4 ± 2.6 | 14.2 ± 3.5 | 13.1 ± 2.7 | 5.4 ± 2.6 | 24.0 ± 3.5 | |
| Y6 | 6.6 ± 1.3 | 1.7 ± 3.6 | 12.0 ± 3.2 | 7.9 ± 2.8 | 2.0 ± 2.8 | 14.2 ± 2.1 | |
| Y7 | 10.5 ± 2.6 | 4.8 ± 0.1 | 19.8 ± 0.1 | 14.0 ± 0.1 | 3.5 ± 0.1 | 32.7 ± 0.1 | |
| Y8 | 10.2 ± 1.9 | 3.5 ± 4.4 | 18.4 ± 2.9 | 10.3 ± 1.3 | 2.8 ± 1.1 | 9.1 ± 2.8 | |
| Y9 | 12.0 ± 3.2 | 11.4 ± 3.2 | 12.9 ± 3.0 | 10.6 ± 2.5 | 2.9 ± 3.0 | 23.5 ± 1.2 | |
| Y10 | 10.4 ± 2.9 | 6.1 ± 2.9 | 9.3 ± 2.9 | 8.5 ± 1.0 | 2.3 ± 1.3 | 20.1 ± 0.8 | |
| Y11 | 10.2 ± 3.2 | 2.5 ± 2.6 | 13.0 ± 3.1 | 11.1 ± 1.2 | 3.3 ± 2.8 | 11.1 ± 1.2 | |
| Y12 | 11.0 ± 3.3 | 15.8 ± 1.1 | 27.2 ± 2.5 | 19.6 ± 2.1 | 4.5 ± 1.6 | 22.3 ± 3.3 | |
| Y13 | 12.5 ± 3.4 | 23.3 ± 3.7 | 17.4 ± 2.4 | 12.5 ± 2.5 | 3.0 ± 2.4 | 18.4 ± 2.6 | |
| Y14 | 19.8 ± 0.7 | 12.6 ± 2.5 | 21.2 ± 1.4 | 17.5 ± 0.2 | 6.0 ± 2.0 | 22.0 ± 0.6 | |
| No. | Paste Yield (%) | pH | Transfer Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Gastrodin | p-Hydroxybenzyl Alcohol | Parishin A | Parishin B | Parishin C | Parishin E | |||
| Y1 | 21.9 ± 1.3 | 3.8 | 108.2 | 254.0 | 53.8 | 75.3 | 70.0 | 58.3 |
| Y2 | 11.7 ± 2.1 | 4.7 | 82.6 | 393.5 | 37.1 | 58.3 | 40.5 | 34.1 |
| Y3 | 18.6 ± 0.9 | 5.7 | 90.5 | 159.3 | 37.8 | 55.6 | 58.9 | 44.4 |
| Y4 | 18.0 ± 1.0 | 5.6 | 47.5 | 124.0 | 33.7 | 32.0 | 27.6 | 17.3 |
| Y5 | 24.4 ±1.2 | 5.6 | 144.8 | 192.6 | 61.5 | 87.7 | 117.3 | 86.3 |
| Y6 | 28.8 ± 2.9 | 5.7 | 115.9 | 213.2 | 49.5 | 87.4 | 90.1 | 84.8 |
| Y7 | 15.7 ± 2.2 | 5.4 | 118.7 | 91.9 | 61.1 | 68.7 | 71.8 | 51.6 |
| Y8 | 24.9 ± 1.6 | 3.0 | 76.4 | 144.6 | 37.6 | 50.1 | 54.6 | 44.7 |
| Y9 | 14.3 ± 2.1 | 4.4 | 94.3 | 300.5 | 50.0 | 85.5 | 109.6 | 128.9 |
| Y10 | 17.2 ± 0.6 | 5.3 | 78.7 | 193.9 | 39.9 | 50.9 | 50.2 | 41.6 |
| Y11 | 19.9 ± 0.3 | 5.3 | 79.8 | 106.3 | 44.9 | 54.4 | 52.3 | 42.4 |
| Y12 | 14.4 ± 1.1 | 5.4 | 100.0 | 126.6 | 32.3 | 70.5 | 81.9 | 113.2 |
| Y13 | 16.2 ± 1.0 | 5.8 | 130.0 | 206.9 | 37.4 | 44.2 | 60.2 | 71.2 |
| Y14 | 16.1 ± 1.8 | 4.3 | 211.4 | 519.4 | 76.1 | 147.4 | 178.7 | 104.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Min, K.; Li, H.; Wang, Y.; Liu, M.; Ma, M.; Zhou, D.; Tu, H.; Chen, B. pH as a Key Factor for the Quality Assurance of the Preparation of Gastrodiae Rhizoma Formula Granules. Molecules 2022, 27, 8091. https://doi.org/10.3390/molecules27228091
Xie S, Min K, Li H, Wang Y, Liu M, Ma M, Zhou D, Tu H, Chen B. pH as a Key Factor for the Quality Assurance of the Preparation of Gastrodiae Rhizoma Formula Granules. Molecules. 2022; 27(22):8091. https://doi.org/10.3390/molecules27228091
Chicago/Turabian StyleXie, Shuting, Ke Min, Hai Li, Ying Wang, Mincong Liu, Ming Ma, Desheng Zhou, Haijun Tu, and Bo Chen. 2022. "pH as a Key Factor for the Quality Assurance of the Preparation of Gastrodiae Rhizoma Formula Granules" Molecules 27, no. 22: 8091. https://doi.org/10.3390/molecules27228091