Enhanced Adsorption of Sulfonamides by Attapulgite-Doped Biochar Prepared with Calcination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Adsorbents
2.3. Characterization of Adsorbent
2.4. Sulfonamides Adsorption Experiments
2.5. Adsorption Kinetic and Thermodynamic
3. Results and Discussion
3.1. Characterization of Adsorbent
3.2. Synthesis of ATP/BC
3.3. Adsorption Performance
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
3.6. Adsorption Thermodynamics
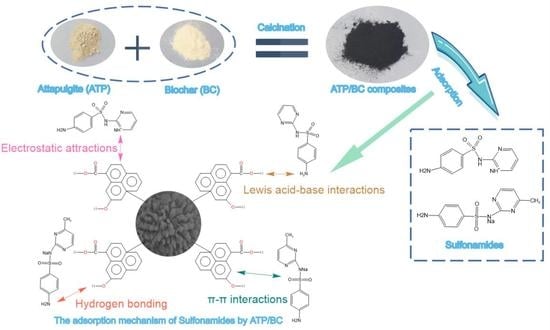
3.7. Potential Adsorption Mechanisms
- (1)
- Electrostatic interactions are an important mechanism to control antibiotic adsorption on carbon materials [45,48]. Under acidic conditions, the surface of sulfonamides binds more hydrogen atoms to present a cationic state and adsorbs with ATP/BC-0.1 by electrostatic interaction. The adsorption effect of ATP/BC-0.1 on sulfonamides is strongly influenced by pH, confirming that electrostatic interactions contribute to the adsorption process.
- (2)
- Hydrogen bonding exists in the adsorption process of polar organic pollutants. The ATP/BC-0.1 surface is rich in hydroxyl groups and the benzene ring is the basic structure of sulfonamides. O–H can act as hydrogen bonding donors to form hydrogen bonds with carbon materials, whereas the benzene rings serve as hydrogen bonding acceptors. The O–H vibration at 3418 cm−1 moves to lower absorbance areas upon adsorption, which can be attributed to the hydrogen bonding interaction between sulfonamide molecules and these functional groups [51], and Lang reached a similar conclusion when studying the adsorption of antibiotics by phosphorylated alkali lignin [52].
- (3)
- π–π interactions are considered to be the driving force for the adsorption of organic chemicals on carbon materials [41]. The amino and sulfonamide groups in sulfonamides act as π-electron acceptors, π orbitals formed by atoms on ATP/BC-0.1 act as π-electron donors, and π–π interactions are generated between ATP/BC-0.1 and sulfonamides for adsorption.
- (4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, D.; Sui, Q.; Mei, X.; Yu, X.; Gu, Y.; Zhao, W. Non-antibiotics matter: Evidence from a one-year investigation of livestock wastewater from six farms in East China. Sci. Total Environ. 2022, 846, 157418. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, S.; Asadipour, A.; Pournamdari, M.; Behnam, B.; Rahimi, H.R.; Dolatabadi, M. Removal of ciprofloxacin from hospital wastewater using electrocoagulation technique by aluminum electrode: Optimization and modelling through response surface methodology. Process Saf. Environ. 2017, 109, 538–547. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Sacher, F.; Lange, F.T.; Brauch, H.J.; Blankenhorn, I. Pharmaceuticals in groundwaters—Analytical methods and results of a monitoring program in Baden-Wurttemberg, Germany. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Pelalak, R.; Alizadeh, R.; Ghareshabani, E.; Heidari, Z. Degradation of sulfonamide antibiotics using ozone-based advanced oxidation process: Experimental, modeling, transformation mechanism and DFT study. Sci. Total Environ. 2020, 734, 139446. [Google Scholar] [CrossRef]
- Meng, L.; Tian, H.; Lv, J.; Wang, Y.; Jiang, G. Influence of microplastics on the photodegradation of perfluorooctane sulfonamide (FOSA). J. Environ. Sci. 2023, 127, 791–798. [Google Scholar] [CrossRef]
- Yao, B.; Luo, Z.; Yang, J.; Zhi, D.; Zhou, Y. Fe(II)Fe(III) layered double hydroxide modified carbon felt cathode for removal of ciprofloxacin in electro-Fenton process. Environ. Res. 2021, 197, 111144. [Google Scholar] [CrossRef]
- Chen, J.; Tong, T.; Jiang, X.; Xie, S. Biodegradation of sulfonamides in both oxic and anoxic zones of vertical flow constructed wetland and the potential degraders. Environ. Pollut. 2020, 265 Pt B, 115040. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; Ferro-Garcia, M.A.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Matsuura, R.; Kanehara, R.; Kadoya, A.; Suzuki, S. Adsorption of sulfonamides to marine diatoms and arthropods. Environ. Toxicol. Pharmacol. 2021, 82, 103557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, Y.; An, B.; Li, L.; Liu, Y. Effect of molecular structure on the adsorption affinity of sulfonamides onto CNTs: Batch experiments and DFT calculations. Chemosphere 2020, 246, 125778. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Lu, J.; Chen, Q.; Chen, C.; Lu, N. High-density Si nanopillars modified with Ag nanoislands: Sensitive SALDI-MS chip for sulfonamides. Sens. Actuators B Chem. 2022, 364, 120890. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, X.; Liu, Y.; Zhou, P.; Ma, G.; Lei, Z.; Lei, L. A low cost and highly efficient adsorbent (activated carbon) prepared from waste potato residue. J. Taiwan Inst. Chem. Eng. 2015, 49, 206–211. [Google Scholar] [CrossRef]
- Lawal, I.A.; Klink, M.; Ndungu, P. Deep eutectic solvent as an efficient modifier of low-cost adsorbent for the removal of pharmaceuticals and dye. Environ. Res. 2019, 179 Pt B, 108837. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Pittman, C.U., Jr.; Mohan, D. Ciprofloxacin and acetaminophen sorption onto banana peel biochars: Environmental and process parameter influences. Environ. Res. 2021, 201, 111218. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Tang, X.; Thiele-Bruhn, S.; Kim, K.H.; Lee, S.E.; Ok, Y.S. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J. Environ. Manag. 2014, 141, 95–103. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.C.; Cho, J.S.; Lee, S.E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. A 2022, 7, 100134. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Fang, J.; Zhang, M.; Chen, H.; Zhou, Y.; Creamer, A.E.; Sun, Y.; Yang, L. Characterization and environmental applications of clay–biochar composites. Chem. Eng. J. 2014, 242, 136–143. [Google Scholar] [CrossRef]
- Dai, L.; Meng, K.; Zhao, W.; Han, T.; Lei, Z.; Ma, G.; Wu, C.; Jia, H. Enhanced removal of Cd2+ by nano zero-valent iron modified attapulgite from aqueous solution: Optimal design, characterization and adsorption mechanism. J. Environ. Chem. Eng. 2022, 10, 107719. [Google Scholar] [CrossRef]
- Zghair, L.A.G.; Hamad, H.H.; Mohamad, S.A.; Al-Hamd, R.K.S. Evaluate the compressive strength of cement paste modified with high reactivity attapulgite and affected by curing temperature. Mater. Today Proc. 2022, 52, 361–366. [Google Scholar] [CrossRef]
- Quan, G.; Sui, F.; Wang, M.; Cui, L.; Wang, H.; Xiang, W.; Li, G.; Yan, J. Mechanochemical modification of biochar-attapulgite nanocomposites for cadmium removal: Performance and mechanisms. BioChem. Eng. J. 2022, 179, 108332. [Google Scholar] [CrossRef]
- Abdulkareem, M.A.; Joudi, M.S.; Ali, A.H. Eco-friendly synthesis of low-cost antibacterial agent (brown attapulgite-Ag nanocomposite) for environmental application. Chem. Data Collect. 2022, 37, 100814. [Google Scholar] [CrossRef]
- Gao, B.; Chang, Q.; Xi, Z.; El-Sayed, M.M.H.; Shoeib, T.; Yang, H. Fabrication of environmentally-friendly composited sponges for efficient removal of fluoroquinolones antibiotics from water. J. Hazard. Mater. 2022, 426, 127796. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, L.; Ge, Y.; Su, H.; Li, Z. Lignin xanthate resin-bentonite clay composite as a highly effective and low-cost adsorbent for the removal of doxycycline hydrochloride antibiotic and mercury ions in water. J. Hazard. Mater. 2019, 368, 33–41. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Xie, X.; Zhu, J.; Li, R.; Qin, T. Removal of Norfloxacin from aqueous solution by clay-biochar composite prepared from potato stem and natural attapulgite. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 126–136. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, Y.; Chen, H.; Liu, Y.; Huang, R.; Pan, J. Biochars derived from by-products of microalgae pyrolysis for sorption of gaseous H2S. J. Environ. Chem. Eng. 2022, 10, 107370. [Google Scholar] [CrossRef]
- Manoharan, T.; Ganeshalingam, S.; Nadarajah, K. Mechanisms of emerging contaminants removal by novel neem chip biochar. Environ. Adv. 2022, 7, 100158. [Google Scholar] [CrossRef]
- Lawal, A.A.; Hassan, M.A.; Zakaria, M.R.; Yusoff, M.Z.M.; Norrrahim, M.N.F.; Mokhtar, M.N.; Shirai, Y. Effect of oil palm biomass cellulosic content on nanopore structure and adsorption capacity of biochar. Bioresour. Technol. 2021, 332, 125070. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Li, L.; Sun, L.; Zhang, S.; Shan, X.-q.; Yang, S.; Wen, B. Adsorption characteristics of 1,2,4-trichlorobenzene, 2,4,6-trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon 2013, 51, 156–163. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Choma, J.; Jagiello, J.; Jaroniec, M. Assessing the contribution of micropores and mesopores from nitrogen adsorption on nanoporous carbons: Application to pore size analysis. Carbon 2021, 183, 150–157. [Google Scholar] [CrossRef]
- Li, K.; Zhou, Y.; Li, J.; Liu, J. Soft-templating synthesis of partially graphitic Fe-embedded ordered mesoporous carbon with rich micropores from bayberry kernel and its adsorption for Pb(II) and Cr(III). J. Taiwan Inst. Chem. Eng. 2018, 82, 312–321. [Google Scholar] [CrossRef]
- Wang, J.; Fu, T.; Meng, F.; Zhao, D.; Chuang, S.S.C.; Li, Z. Highly active catalysis of methanol oxidative carbonylation over nano Cu2O supported on micropore-rich mesoporous carbon. Appl. Catal. B Environ. 2022, 303, 120890. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, S.; Li, L.; Li, C.; Zhang, S.; Shan, X.Q.; Wen, B.; Guo, B. Effects of copper and aluminum on the adsorption of sulfathiazole and tylosin on peat and soil. Environ. Pollut. 2014, 184, 579–585. [Google Scholar] [CrossRef]
- Hamadeen, H.M.; Elkhatib, E.A. New nanostructured activated biochar for effective removal of antibiotic ciprofloxacin from wastewater: Adsorption dynamics and mechanisms. Environ. Res. 2022, 210, 112929. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Jung, C.; Li, H.; Yu, M.; Flora, J.R.; Boateng, L.K.; Her, N.; Zoh, K.D.; Yoon, Y. Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 2015, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Huang, G.; Yao, Y.; An, C.; Zhang, P.; Zhao, K. Investigation into the influencing factors and adsorption characteristics in the removal of sulfonamide antibiotics by carbonaceous materials. J. Clean. Prod. 2021, 319, 128692. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Al-Jendeel, H.A.; Rashid, S.A.; Al-Batty, S. Antibiotics adsorption from contaminated water by composites of ZSM-5 zeolite nanocrystals coated carbon. J. Water Pro. Eng. 2022, 47, 102745. [Google Scholar] [CrossRef]
- Atugoda, T.; Gunawardane, C.; Ahmad, M.; Vithanage, M. Mechanistic interaction of ciprofloxacin on zeolite modified seaweed (Sargassum crassifolium) derived biochar: Kinetics, isotherm and thermodynamics. Chemosphere 2021, 281, 130676. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Siahpoosh, Z.H. Ghezeljeh nanoclay as a new natural adsorbent for the removal of copper and mercury ions: Equilibrium, kinetics and thermodynamics studies. Chin. J. Chem. Eng. 2015, 23, 1819–1833. [Google Scholar] [CrossRef]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef]
- Keerthanan, S.; Bhatnagar, A.; Mahatantila, K.; Jayasinghe, C.; Ok, Y.S.; Vithanage, M. Engineered tea-waste biochar for the removal of caffeine, a model compound in pharmaceuticals and personal care products (PPCPs), from aqueous media. Environ. Technol. Innov. 2020, 19, 100847. [Google Scholar] [CrossRef]
- Guo, X.; Dong, H.; Yang, C.; Zhang, Q.; Liao, C.; Zha, F.; Gao, L. Application of goethite modified biochar for tylosin removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 81–88. [Google Scholar] [CrossRef]
- Hettithanthri, O.; Rajapaksha, A.U.; Keerthanan, S.; Ramanayaka, S.; Vithanage, M. Colloidal biochar for enhanced adsorption of antibiotic ciprofloxacin in aqueous and synthetic hydrolyzed human urine matrices. Chemosphere 2022, 297, 133984. [Google Scholar] [CrossRef]
- Ye, S.; Yan, M.; Tan, X.; Liang, J.; Zeng, G.; Wu, H.; Song, B.; Zhou, C.; Yang, Y.; Wang, H. Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Appl. Catal. B Environ. 2019, 250, 78–88. [Google Scholar] [CrossRef]
- Gong, L.; Wu, H.; Shan, X.; Li, Z. Facile fabrication of phosphorylated alkali lignin microparticles for efficient adsorption of antibiotics and heavy metal ions in water. J. Environ. Chem. Eng. 2021, 9, 106574. [Google Scholar] [CrossRef]









| Sample | Specific Surface Area (m2∙g−1) | Adsorption Average Pore Size (nm) |
|---|---|---|
| BC | 49.20 | 6.61 |
| ATP | 71.91 | 8.27 |
| ATP/BC-0.01 | 97.33 | 4.17 |
| ATP/BC-0.1 | 113.75 | 3.01 |
| ATP/BC-0.3 | 97.36 | 4.26 |
| ATP/BC-0.5 | 85.36 | 4.84 |
| Category | Model Parameters | SD | SMZ |
|---|---|---|---|
| Pseudo-first-order kinetic | qe (mg∙g−1) | 0.66 | 0.95 |
| k1 (min−1) | 1.01 × 10−2 | 1.43 × 10−2 | |
| R2 | 0.93 | 0.79 | |
| Pseudo-second-order kinetic | qe (mg∙g−1) | 3.55 | 3.49 |
| k2 (g∙(mg∙min)−1) | 11.01 | 9.60 | |
| R2 | 0.99 | 0.99 | |
| Elovich kinetic | α (mg∙g−1) | 1.07 × 10−1 | 2.21 × 10−1 |
| β (g∙(mg∙min)−1) | 2.92 | 2.32 | |
| R2 | 0.93 | 0.97 | |
| Diffusion | kd1 (mg∙g−1∙min−0.5) | 7.01 × 10−1 | 9.75 × 10−1 |
| C1 (mg∙g−1) | 2.37 | 1.48 | |
| R12 | 0.94 | 0.95 | |
| kd2 (mg∙g−1∙min−0.5) | 3.69 × 10−2 | 5.97 × 10−2 | |
| C2 (mg∙g−1) | 3.02 | 2.68 | |
| R22 | 0.96 | 0.74 |
| Sulfonamides | Temperature | Freundlich Isotherm | Langmuir Isotherm | ||||
|---|---|---|---|---|---|---|---|
| n | kf | R2 | qm (mg∙g−1) | kl | R2 | ||
| SD | 20 °C | 1.69 | 1.83 | 0.99 | 7.74 | 0.37 | 0.86 |
| 30 °C | 1.84 | 2.07 | 0.99 | 7.20 | 0.51 | 0.89 | |
| 40 °C | 2.03 | 2.31 | 0.99 | 7.23 | 0.59 | 0.87 | |
| SMZ | 20 °C | 1.90 | 1.69 | 0.99 | 5.89 | 0.53 | 0.97 |
| 30 °C | 2.19 | 2.04 | 0.99 | 5.91 | 0.71 | 0.94 | |
| 40 °C | 2.37 | 2.28 | 0.98 | 5.73 | 1.01 | 0.96 | |
| Types of Antibiotics | ΔG (kJ∙mol−1) | ΔH (kJ∙mol−1) | ΔS (J∙(mol∙K)−1) | ||
|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | |||
| SD | −23.73 | −25.07 | −26.12 | 8.79 | 111.61 |
| SMZ | −23.92 | −25.04 | −26.15 | 11.39 | 119.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Liu, F.; Shan, Y.; Huang, Z.; Gao, J.; Jiao, W. Enhanced Adsorption of Sulfonamides by Attapulgite-Doped Biochar Prepared with Calcination. Molecules 2022, 27, 8076. https://doi.org/10.3390/molecules27228076
Hu J, Liu F, Shan Y, Huang Z, Gao J, Jiao W. Enhanced Adsorption of Sulfonamides by Attapulgite-Doped Biochar Prepared with Calcination. Molecules. 2022; 27(22):8076. https://doi.org/10.3390/molecules27228076
Chicago/Turabian StyleHu, Jianqiao, Feng Liu, Yongping Shan, Zhenzhen Huang, Jingqing Gao, and Wentao Jiao. 2022. "Enhanced Adsorption of Sulfonamides by Attapulgite-Doped Biochar Prepared with Calcination" Molecules 27, no. 22: 8076. https://doi.org/10.3390/molecules27228076