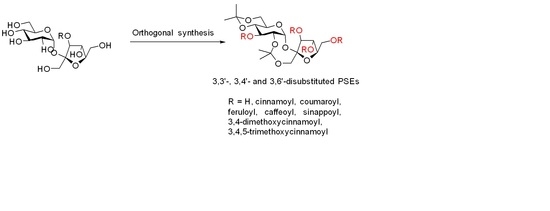
Targeted Synthesis of 3,3′-, 3,4′- and 3,6′-Phenylpropanoid Sucrose Esters
Abstract
:1. Introduction
2. Results and Discussion
3. Synthesis of 3,3′-di-Substituted PSEs
4. Synthesis of 3,4′-di-Substituted PSEs
5. Synthesis of 3,6′-di-Substituted PSEs
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Panda, P.; Appalashetti, M.; Judeh, Z.M.A. Phenylpropanoid sucrose esters: Plant-derived natural products as potential leads for new therapeutics. Curr. Med. Chem. 2011, 18, 3234–3251. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Terrier, L.; Hay, A.-E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F.Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Kumagai, H.; Kurihara, H.; Yamazaki, K.; Sawano, R.; Inoue, N. β-Glucosidase inhibitory activities of phenylpropanoid glycosides, vanicoside A and B from Polygonum sachalinense rhizome. Fitoterapia 2006, 77, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yip, Y.M.; Song, L.; Feng, S.; Liu, Y.; Lai, F.; Zhang, D.; Huang, D. Inhibiting enzymatic starch digestion by the phenolic compound diboside A: A mechanistic and in silico study. Food Res. Int. 2013, 54, 595–600. [Google Scholar] [CrossRef]
- Surabhi, D.R. Studies on Bioactivity of Phenylpropanoid Sucrose Esters. Ph.D. Thesis, Nanyang Technological University, Singapore, 2017. [Google Scholar]
- Lin, O.L. Development of Phenylpropanoid Sucrose Esters as Antidiabetic Lead Compounds. Ph.D. Thesis, Nanyang Technological University, Interdisciplinary Graduate School (NTU Institute for Health Technologies), Singapore, 2018. [Google Scholar]
- Wong, P.W.K. Investigation of Phenylpropanoid Sucrose Esters as Antidiabetic Compounds. Ph.D. Thesis, Nanyang Technological University, Interdisciplinary Graduate School (NTU Institute for Health Technologies), Singapore, 2020. [Google Scholar]
- Devaraj, S.; Yip, Y.M.; Panda, P.; Ong, L.L.; Wong, P.W.K.; Zhang, D.; Ali, Y.; Judeh, Z. Feruloyl Sucrose Esters: Potent and Selective Inhibitors of α-glucosidase and α-amylase. Curr. Med. Chem. 2021, 68, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Yip, Y.M.; Panda, P.; Ong, L.L.; Wong, P.W.K.; Zhang, D.; Ali, Y.; Judeh, Z. Cinnamoyl Sucrose Esters as Alpha Glucosidase Inhibitors for the Treatment of Diabetes. Molecules 2021, 26, 469. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.; O’Keefe, J.H.; Jellinger, P. Postprandial dysmetabolism: The missing link between diabetes and cardiovascular events? Endocr. Pract. 2008, 14, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Muraoka, T.; Ito, Y.; Tagashi, Y.; Teraushi, Y. Comparison of adverse gastrointestinal effects of acarbose and miglitol in healthy men: A crossover study. Intern. Med. 2010, 49, 1085–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, R.G. Combination therapy for patients with type 2 diabetes: Repaglinide in combination with metformin. Expert Rev. Endocrinol. Metab. 2010, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Bidot, P.; Freedman, Z.; Haag, B.; Podlecki, D.; Rendell, M.; Schimel, D.; Weiss, S.; Taylor, T.; Krol, A.; et al. Efficacy and safety of acarbose in insulin-treated patients with type 2 diabetes. Diabetes Care 1998, 21, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Appalashetti, M.; Natarajan, M.; Mary, C.-P.; Venkatraman, S.S.; Judeh, Z.M.A. Synthesis and antiproliferative activity of helonioside A, 3′,4′,6′-tri-O-feruloylsucrose, lapathoside C and their analogs. Eur. J. Med. Chem. 2012, 58, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Appalashetti, M.; Natarajan, M.; Chan-Park, M.B.; Venkatraman, S.S.; Judeh, Z.M.A. Synthesis and antitumor activity of lapathoside D and its analogs. Eur. J. Med. Chem. 2012, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poschalko, A.; Rohr, T.; Gruber, H.; Bianco, A.; Guichard, G.; Briand, J.-P.; Weber, V.; Falkenhagen, D. SUBPOL: A Novel Sucrose-Based Polymer Support for Solid-Phase Peptide Synthesis and Affinity Chromatography Applications. J. Am. Chem. Soc. 2003, 125, 13415–13426. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.-I.; Sakai, K.; Tsushima, K.; Akai, S. The first total synthesis of telephiose A. Tetrahedron Lett. 2007, 48, 3745–3748. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathy, W.P.W.; Ong, L.L.; Devaraj, S.; Khong, D.T.; Judeh, Z.M.A. Targeted Synthesis of 3,3′-, 3,4′- and 3,6′-Phenylpropanoid Sucrose Esters. Molecules 2022, 27, 535. https://doi.org/10.3390/molecules27020535
Kathy WPW, Ong LL, Devaraj S, Khong DT, Judeh ZMA. Targeted Synthesis of 3,3′-, 3,4′- and 3,6′-Phenylpropanoid Sucrose Esters. Molecules. 2022; 27(2):535. https://doi.org/10.3390/molecules27020535
Chicago/Turabian StyleKathy, Wong Pooi Wen, Li Lin Ong, Surabhi Devaraj, Duc Thinh Khong, and Zaher M. A. Judeh. 2022. "Targeted Synthesis of 3,3′-, 3,4′- and 3,6′-Phenylpropanoid Sucrose Esters" Molecules 27, no. 2: 535. https://doi.org/10.3390/molecules27020535