A Simple Model to Estimate the Number of Metal Engineered Nanoparticles in Samples Using Inductively Coupled Plasma Optical Emission Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Characterization of Engineered Nanoparticles
2.2. Analysis of Gold Salts and Nanoparticles
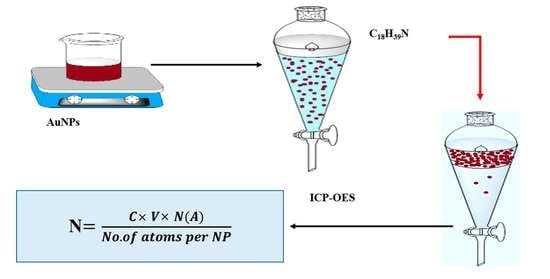
2.3. Extraction and Separation Methods of AuNPs
2.4. Modeling of Nanoparticles
3. Materials and Methods
3.1. Chemicals, Reagents, and Standards
3.2. Instruments
3.3. Preparation of Metal Standards Solutions and Nanomaterials Stock Solutions
3.4. Sampling and Sample Preparation
3.5. Extraction of AuNPs in Sediments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chekli, L.; Zhao, Y.X.; Tijing, L.D.; Phuntsho, S.; Donner, E.; Lombi, E.; Gao, B.Y.; Shon, H.K. Aggregation behaviour of engineered nanoparticles in natural waters: Characterising aggregate structure using on-line laser light scattering. J. Hazard. Mater. 2015, 284, 190–200. [Google Scholar] [CrossRef]
- Domingos, R.F.; Baalousha, M.A.; Ju-Nam, Y.; Reid, M.M.; Tufenkji, N.; Lead, J.R.; Leppard, G.G.; Wilkinson, K.J. Characterizing Manufactured Nanoparticles in the Environment: Multimethod Determination of Particle Sizes. Environ. Sci. Technol. 2009, 43, 7277–7284. [Google Scholar] [CrossRef]
- Bolaños-Benítez, V.; McDermott, F.; Gill, L.; Knappe, J. Engineered silver nanoparticle (Ag-NP) behaviour in domestic on-site wastewater treatment plants and in sewage sludge amended-soils. Sci. Total Environ. 2020, 722, 137794. [Google Scholar] [CrossRef]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Smith, M.E.; Rogers, K.; Patra, M.; Hammer, K.A.; Allen, H.J.; Vulpe, C.D. Differential Gene Expression in Daphnia magna Suggests Distinct Modes of Action and Bioavailability for ZnO Nanoparticles and Zn Ions. Environ. Sci. Technol. 2010, 45, 762–768. [Google Scholar] [CrossRef]
- Chae, Y.J.; Pham, C.H.; Lee, J.; Bae, E.; Yi, J.; Gu, M.B. Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2009, 94, 320–327. [Google Scholar] [CrossRef]
- Handy, R.D.; von der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef]
- Gao, J.; Youn, S.; Hovsepyan, A.; Llaneza, V.L.; Wang, Y.; Bitton, G.; Bonzongo, J.-C.J. Dispersion and Toxicity of Selected Manufactured Nanomaterials in Natural River Water Samples: Effects of Water Chemical Composition. Environ. Sci. Technol. 2009, 43, 3322–3328. [Google Scholar] [CrossRef]
- Scown, T.; van Aerle, R.; Tyler, C. Do engineered nanoparticles pose a significant threat to the aquatic environment? Crit. Rev. Toxicol. 2010, 40, 653–670. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Zhang, D.; Qiu, J.; Shi, L.; Liu, Y.; Pan, B.; Xing, B. The mechanisms and environmental implications of engineered nanoparticles dispersion. Sci. Total Environ. 2020, 722, 137781. [Google Scholar] [CrossRef]
- Bathi, J.R.; Moazeni, F.; Upadhyayula, V.K.; Chowdhury, I.; Palchoudhury, S.; Potts, G.E.; Gadhamshetty, V. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci. Total Environ. 2021, 793, 148560. [Google Scholar] [CrossRef]
- Shang, J.; Gao, X. Nanoparticle counting: Towards accurate determination of the molar concentration. Chem. Soc. Rev. 2014, 43, 7267–7278. [Google Scholar] [CrossRef]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.; Chen, A.H.; Huo, Q. A One-Step Homogeneous Immunoassay for Cancer Biomarker Detection Using Gold Nanoparticle Probes Coupled with Dynamic Light Scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef]
- Reipa, V.; Purdum, G.; Choi, J. Measurement of Nanoparticle Concentration Using Quartz Crystal Microgravimetry. J. Phys. Chem. B 2010, 114, 16112–16117. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Levin, A.D.; Nagaev, A.I.; Sadagov, A.Y. Determination of Number Density of Particles Together with Measurement of Their Sizes by Dynamic Light Scattering. Meas. Tech. 2018, 61, 760–766. [Google Scholar] [CrossRef]
- Yang, Y.; Long, C.-L.; Li, H.-P.; Wang, Q.; Yang, Z.-G. Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci. Total Environ. 2016, 563–564, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, J.; Praetorius, A.; Gondikas, A.; Fabienke, W.; von der Kammer, F.; Hofmann, T. Detection of Engineered Copper Nanoparticles in Soil Using Single Particle ICP-MS. Int. J. Environ. Res. Public Health 2015, 12, 15020. [Google Scholar] [CrossRef]
- Gondikas, A.; von der Kammer, F.; Kaegi, R.; Borovinskaya, O.; Neubauer, E.; Navratilova, J.; Praetorius, A.; Cornelis, G.; Hofmann, T. Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters. Environ. Sci. Nano 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Donovan, A.R.; Adams, C.D.; Ma, Y.; Stephan, C.; Eichholz, T.; Shi, H. Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere 2016, 144, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, D.M.; Lesher, E.K.; Bednar, A.; Monserud, J.; Higgins, C.P.; Ranville, J.F. Detecting nanoparticulate silver using single-particle inductively coupled plasma-mass spectrometry. Nanomater. Environ. 2012, 31, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-j.; Shih, Y.-h.; Su, C.-H.; Ho, H.-C. Comparison of three analytical methods to measure the size of silver nanoparticles in real environmental water and wastewater samples. J. Hazard. Mater. 2017, 322, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Aznar, R.; Barahona, F.; Geiss, O.; Ponti, J.; Luis, T.J.; Barrero-Moreno, J. Quantification and size characterisation of silver nanoparticles in environmental aqueous samples and consumer products by single particle-ICPMS. Talanta 2017, 175, 200–208. [Google Scholar] [CrossRef]
- Donahue, N.D.; Francek, E.R.; Kiyotake, E.; Thomas, E.E.; Yang, W.; Wang, L.; Detamore, M.S.; Wilhelm, S. Assessing nanoparticle colloidal stability with single-particle inductively coupled plasma mass spectrometry (SP-ICP-MS). Anal. Bioanal. Chem. 2020, 412, 5205–5216. [Google Scholar] [CrossRef]
- Bocca, B.; Battistini, B.; Petrucci, F. Silver and gold nanoparticles characterization by SP-ICP-MS and AF4-FFF-MALS-UV-ICP-MS in human samples used for biomonitoring. Talanta 2020, 220, 121404. [Google Scholar] [CrossRef]
- Fréchette-Viens, L.; Hadioui, M.; Wilkinson, K.J. Quantification of ZnO nanoparticles and other Zn containing colloids in natural waters using a high sensitivity single particle ICP-MS. Talanta 2019, 200, 156–162. [Google Scholar] [CrossRef]
- Zhao, J.; Friedrich, B. Synthesis of Gold Nanoparticles Via the Chemical Reduction Methods. In Proceedings of the 7th International Conference on Nanomaterials-Research & Application, Hotel Voronez I, Brno, Czech Republic, 14–16 October 2015; pp. 597–604. [Google Scholar]
- Alaqad, K.; Saleh, T.A. Gold and silver nanoparticles: Synthesis methods, characterization routes and applications towards drugs. J. Environ. Anal. Toxicol. 2016, 6, 525–2161. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Gumbi, B.; Ngila, J.C.; Ndungu, P.G. Gold nanoparticles for the quantification of very low levels of poly-diallyldimethylammonium chloride in river water. Anal. Methods 2014, 6, 6963–6972. [Google Scholar] [CrossRef]
- Milam, S. Effects of Silver Nanoparticles on Photochemical Processes Focusing on Luminol Chemiluminescence. Master’s Thesis, Eastern Michigan University, Ypsilanti, MI, USA, 2010. [Google Scholar]
- Ziegler, C.; Eychmüller, A. Seeded Growth Synthesis of Uniform Gold Nanoparticles with Diameters of 15−300 nm. J. Phys. Chem. C 2011, 115, 4502–4506. [Google Scholar] [CrossRef]
- Leng, W.N.; Pati, P.; Vikesland, P.J. Room temperature seed mediated growth of gold nanoparticles: Mechanistic investigations and life cycle assesment. Environ. Sci.-Nano. 2015, 2, 440–453. [Google Scholar] [CrossRef]
- Du, H. Mie-scattering calculation. Appl. Opt. 2004, 43, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Keener, J.D.; Chalut, K.J.; Pyhtila, J.W.; Wax, A. Application of Mie theory to determine the structure of spheroidal scatterers in biological materials. Opt. Lett. 2007, 32, 1326–1328. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. Determination of Size and Concentration of Gold Nanoparticles from Extinction Spectra. Anal. Chem. 2008, 80, 6620–6625. [Google Scholar] [CrossRef]
- Niskanen, I.; Forsberg, V.; Zakrisson, D.; Reza, S.; Hummelgård, M.; Andres, B.; Fedorov, I.; Suopajärvi, T.; Liimatainen, H.; Thungström, G. Determination of nanoparticle size using Rayleigh approximation and Mie theory. Chem. Eng. Sci. 2019, 201, 222–229. [Google Scholar] [CrossRef]
- Postelmans, A.; Aernouts, B.; Saeys, W. Estimation of particle size distributions from bulk scattering spectra: Sensitivity to distribution type and spectral noise. Opt. Express 2018, 26, 15015–15038. [Google Scholar] [CrossRef]
- da Silva, B.F.; Pérez, S.; Gardinalli, P.; Singhal, R.; Mozeto, A.A.; Barceló, D. Analytical chemistry of metallic nanoparticles in natural environments. TrAC Trends Anal. Chem. 2011, 30, 528–540. [Google Scholar] [CrossRef]
- El Hadri, H.; Louie, S.M.; Hackley, V.A. Assessing the interactions of metal nanoparticles in soil and sediment matrices—A quantitative analytical multi-technique approach. Environ. Sci. Nano 2017, 5, 203–214. [Google Scholar] [CrossRef]
- Cui, P.; He, H.; Chen, D.; Liu, H.; Zhang, S.; Yang, J. Phase transfer of noble metal nanoparticles from ionic liquids to an organic/aqueous medium. Ind. Eng. Chem. Res. 2014, 53, 15909–15916. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, E. Size-dependent phase transfer of gold nanoparticles from water into toluene by tetraoctylammonium cations: A wholly electrostatic interaction. J. Phys. Chem. B 2004, 108, 24–26. [Google Scholar] [CrossRef]
- Gittins, D.I.; Caruso, F. Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angewandte Chem. Int. Ed. 2001, 40, 3001–3004. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Gray, E.P.; Higgins, C.P.; Ranville, J.F. Single particle inductively coupled plasma-mass spectrometry: A performance evaluation and method comparison in the determination of nanoparticle size. Environ. Sci. Technol. 2012, 46, 12272–12280. [Google Scholar] [CrossRef] [PubMed]













| AuNPs | ||||
|---|---|---|---|---|
| Reducing Agent | Ascorbic Acid | Tri-Sodium Citrate | Sodium Borohydride | Seed Growth |
| Average diameter nm | 5–15 | 20–30 | 5–10 | 65–80 |
| λmax | 524.94 | 532.38 | 520.95 | 562.03 |
| Volume µL | Concentration mM | Mass µg | No. of NPs |
|---|---|---|---|
| 10 | 0.016624 | 0.032750 | 164,478,413.8 |
| 50 | 0.074379 | 0.73263 | 3,679,510,010 |
| 100 | 0.15330 | 3.0200 | 15,167,634,649 |
| 200 | 0.29613 | 11.668 | 58,598,379,921 |
| 300 | 0.50264 | 29.706 | 1.49193 × 1011 |
| 400 | 0.61058 | 48.113 | 2.4164 × 1011 |
| Instrument | Model | Nanoparticle | Parameter | Extraction Method | Reference |
|---|---|---|---|---|---|
| ICP-MS | Analysis of different fractions | Ti, Ag | Concentration | Centrifuge, evaporation, ultrasonic | Polesel et al. [45] |
| Sp-ICP-MS ICP-MS | NNP = [fNP/9Qsam × ηn)] | Ag | Concentration | Centrifuge | Aznar et al. [23] |
| NNP = [fNP/9Qsam × ηn)] | Au, Ag | Size distribution, particle number concentration, diameter | Centrifuge, ultrasonication | Yang et al. [17] | |
| NNP = [fNP/9Qsam × ηn)] | Ag | Nanoparticle size, | Centrifuge | Pace et al. [46] | |
| Microgravimetry TEM | Cm = ∆mS/υ | Si | Concentration, extinction coefficient, | Reipa et al. [14] | |
| UV-vis TEM | A = πR2Qextd0N/2.303 | Au | Size, concentration, absorbance | Haiss et al. [36] | |
| UV-vis TEM | C = A/ԑd0 | Ag | Concentration, extinction coefficient, size, absorbance | Paramelle et al. [15] | |
| DLS TEM | N(d) = nb – C1 + C2/dn | Ag, Si | Number density, extinction | Levin et al. [16] | |
| UV-vis TEM | C = (α/N) | Nanosphere Polystyrene | Diameter, volume, concentration | Niskanen et al. [39] | |
| ICP-OES, HRTEM, UV-vis | N = (CxNa × V)/Nparticles | Au | Concentration, number of AuNPs, mass of AuNPs, diameter | Leachate, centrifuge, phase transfer | Current method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendricks, N.; Olatunji, O.; Gumbi, B. A Simple Model to Estimate the Number of Metal Engineered Nanoparticles in Samples Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2022, 27, 5810. https://doi.org/10.3390/molecules27185810
Hendricks N, Olatunji O, Gumbi B. A Simple Model to Estimate the Number of Metal Engineered Nanoparticles in Samples Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules. 2022; 27(18):5810. https://doi.org/10.3390/molecules27185810
Chicago/Turabian StyleHendricks, Nokwanda, Olatunde Olatunji, and Bhekumuzi Gumbi. 2022. "A Simple Model to Estimate the Number of Metal Engineered Nanoparticles in Samples Using Inductively Coupled Plasma Optical Emission Spectrometry" Molecules 27, no. 18: 5810. https://doi.org/10.3390/molecules27185810