Nutrient Recovery from Digestate of Agricultural Biogas Plants: A Comparative Study of Innovative Biocoal-Based Additives in Laboratory and Full-Scale Experiments
Abstract
:1. Introduction
2. Materials and Methods
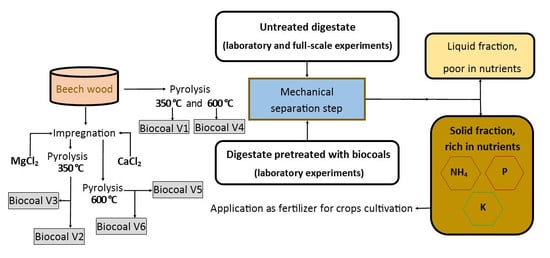
2.1. Experiment Overview
2.1.1. Mechanical Separation Step in the Full-Scale Application
2.1.2. Mechanical Separation Step in Laboratory Conditions
2.1.3. Pretreatment with Additives before the Mechanical Separation Step
2.2. Production of Biocoals
2.3. Analysis of Biocoals
2.3.1. Specific Water Uptake Analysis
2.3.2. Analysis of Biocoals
2.4. Experimental Design
2.5. Analytical Methods
2.6. Calculation of Removal Efficiency
2.7. Calculation of Sorption Capacity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Results of the Mechanical Separation Step without Pretreatment: Comparison of Full-Scale and Laboratory Experiments
3.2. Results of the Laboratory Analysis of the Biocoals
3.3. Water Uptake of the Biocoals
3.4. Results on Nutrients Recovery after Pretreatment
3.4.1. FM-Removal, DM Concentration and DM-Removal Efficiency
3.4.2. NH4 Concentration and NH4-Removal Efficiency
3.4.3. P Concentration and P-Removal Efficiency
3.4.4. K-Concentration and K-Removal Efficiency
3.5. Comparison of the Control Variant with Those Pretreated with Additives with the Best Performance
3.6. Summary of Results and Further Needed Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Global Demand for Agricultural Fertilizer by Nutrient from 2011/2012 to 2021/2022 (in Million Metric tons). Available online: https://www.statista.com/statistics/438930/fertilizer-demand-globally-by-nutrient/ (accessed on 24 January 2022).
- Statista. Phosphate Rock Reserves Worldwide in 2020, by Country (in Million Metric Tons). Available online: https://www.statista.com/statistics/681747/phosphate-rock-reserves-by-country/ (accessed on 24 January 2022).
- Eurostat. Consumption of Inorganic Fertilizers: Nutrient: Nitrogen. Available online: https://ec.europa.eu/eurostat/databrowser/view/AEI_FM_USEFERT/default/table?lang=en&category=agr.aei.aei_nut (accessed on 16 August 2022).
- Eurostat. Air Pollutants by Source Sector. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_air_emis/default/table?lang=en&category=agr.aei.aei_nut (accessed on 16 August 2022).
- Eurostat. Greenhouse Gas Emissions by Source Sector. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_air_gge/default/table?lang=en&category=agr.aei.aei_nut (accessed on 16 August 2022).
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient removal and recovery from digestate: A review of the technology. Biofuels 2018, 9, 247–262. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Biofuels—Status and Perspective. Chapter 24: Phosphorus Removal and Recovery from Digestate after Biogas Production; Biernat, K., Ed.; InTech: Rijeka, Croatia, 2015; ISBN 978-953-51-2177-0. [Google Scholar]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid—liquid separation of animal slurry in theory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Fachverband Biogas, e.V.; Digestate as Ferilizer. November 2018. Available online: https://www.biogas.org/edcom/webfvb.nsf/id/BJHCPA-DE-Digestate-as-Fertilizer (accessed on 16 August 2022).
- Olugbemide, A.D.; Likozar, B. Assessment of Liquid and Solid Digestates from Anaerobic Digestion of Rice Husk as Potential Biofertilizer and Nutrient Source for Microalgae Cultivation. Processes 2022, 10, 1007. [Google Scholar] [CrossRef]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Michels, E.; Meers, E. The Potential of Digestate and the Liquid Fraction of Digestate as Chemical Fertiliser Substitutes under the RENURE Criteria. Agronomy 2021, 11, 1374. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of Liquid Digestate Treatment on Sustainable Microalgae Biomass Production. Bioenerg. Res. 2022, 15, 357–370. [Google Scholar] [CrossRef]
- Zielińska, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Kazimierowicz, J.; Quattrocelli, P.; Dębowski, M. Liquid fraction of digestate pretreated with membrane filtration for cultivation of Chlorella vulgaris. Waste Manag. 2022, 146, 1–10. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci. Total Environ. 2017, 599–600, 1885–1894. [Google Scholar] [CrossRef]
- Rasse, D.P.; Weldon, S.; Joner, E.J.; Joseph, S.; Kammann, C.I.; Liu, X.; O’Toole, A.; Pan, G.; Kocatürk-Schumacher, N.P. Enhancing plant N uptake with biochar-based fertilizers: Limitation of sorption and prospects. Plant Soil 2022, 475, 213–236. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A.A. comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Negi, S.; Jaswal, G.; Dass, K.; Mazumder, K.; Elumalai, S.; Roy, J.K. Torrefaction: A sustainable method for transforming of agri-wastes to high energy density solids (biocoal). Rev. Environ. Sci. Biotechnol. 2020, 19, 463–488. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Arora, A.; Gupta, A.; Saeed, M.A.; Niedzwiecki, L.; Andrews, G.; Phylaktou, H.; Gibbs, B.; Newlaczyl, A.; Livesey, P.M. Biocoal—Quality control and assurance. Biomass Bioenergy 2020, 135, 105509. [Google Scholar] [CrossRef]
- Kalus, K.; Koziel, J.; Opaliński, S. A Review of Biochar Properties and Their Utilization in Crop Agriculture and Livestock Production. Appl. Sci. 2019, 9, 3494. [Google Scholar] [CrossRef]
- Kruse, A.; Zevaco, T. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca–Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef]
- Kocatürk-Schumacher, N.P. Recovery of Nutrients from Biogas Digestate with Biochar and Clinoptilolite. Ph.D Thesis, University of Copenhagen, Copenhagen, Denmark, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4(+)), nitrate (NO3(-)), and phosphate (PO4(3-)). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wen, H.; Zhang, H.; Li, Y.; Mai, X.; Zhang, Y.; Wang, J.; Li, Y.; Zhang, Z. Biogas residue biochar integrated with phosphate from its ash for the effective recovery of nutrients from piggery biogas slurry. Biochar 2022, 4, 374. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.-S. Valorisation of post-sorption materials: Opportunities, strategies, and challenges. Adv. Colloid Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Le, F.; Li, j.-s.; Donatello, S.; Cheeseman, C.R.; Poon, C.S.; Tsang, D.C.W. Use of Mg/Ca modified biochars to take up phosphorus from acid-extract of incinerated sewage sludge ash (ISSA) for fertilizer application. J. Clean. Prod. 2020, 244, 118853. [Google Scholar] [CrossRef]
- Li, S. Combustion synthesis of porous MgO and its adsorption properties. Int. J. Ind. Chem. 2019, 10, 89–96. [Google Scholar] [CrossRef]
- Naegele, H.-J.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Electric Energy Consumption of the Full-Scale Research Biogas Plant “Unterer Lindenhof”: Results of Longterm and Full Detail Measurements. Energies 2012, 5, 5198–5214. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H. Enhancement of methane production with horse manure supplement and pretreatment in a full-scale biogas process. Energy 2014, 73, 523–530. [Google Scholar] [CrossRef]
- Ohnmacht, B.; Kress, P.; Oechsner, H.; Lemmer, A. Investigation of the mixing behaviour of a full-scale biogas plant using biodegradable tracers. Biomass Bioenergy 2020, 139, 105613. [Google Scholar] [CrossRef]
- Vodegel, S. Final Report on the joint Industrial Research Project “Increasing the Economic Efficiency of Biomass CHP Plants Based on Pyrolysis, Gasification and Combustion Processes by Increasing Fuel Flexibility and a Process-Oriented Evaluation of Biomass” (from German: Schlussbericht zu dem industriellen Gemeinschaftsforschung-Vorhaben “Steigerung der Wirtschaftlichkeit von Biomasseheizkraftwerken auf der Basis von Pyrolyse-, Vergasungs- und Verbrennungsverfahren durch Erhöhung der Brennstoffflexibilität und eine prozessorientierte Bewertung der Biomasse“). 2014. Available online: https://www.cutec.de/fileadmin/Cutec/documents/Thermische-Prozesstechnik/Abschlussberichte/AiF_Wirtschaftlichkeit_Biomasseheizkraftwerke_140930.pdf (accessed on 16 August 2022).
- Vodegel, S.; Müller, F. Basic Study of the German Federal Environmental Foundation: Technology Assessment of Thermochemical Conversion Processes of Sewage Sludge as an Alternative to Incineration with Special Consideration of the Potential for Nutrient Recovery (From German: “Deutsche Bundesstiftung Umwelt”-Grundsatzstudie: Technologiebewertung thermochemischer Konversionsverfahren von Klärschlamm als Alternative zur Verbrennung unter besonderer Berücksichtigung der Potenziale zur Nährstoffrückgewinnung). 2017. Available online: https://www.cutec.de/fileadmin/Cutec/documents/Thermische-Prozesstechnik/Abschlussberichte/DBU-Grundsatzstudie_Klaerschlamm_171030.pdf (accessed on 16 August 2022).
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Hsu, J.C.; Peruggia, M. Graphical Representations of Tukey’s Multiple Comparison Method. J. Comput. Graph. Stat. 1994, 3, 143–161. [Google Scholar]
- Piepho, H.-P. Letters in Mean Comparisons: What They Do and Don’t Mean. Agron. J. 2018, 110, 431–434. [Google Scholar] [CrossRef]
- He, Q.; Li, X.; Ren, Y. Analysis of the simultaneous adsorption mechanism of ammonium and phosphate on magnesium-modified biochar and the slow release effect of fertiliser. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]









| Variant | Additive, in g | Reaction Time | |||||
|---|---|---|---|---|---|---|---|
| 5 min | 1 h | 3 h | 20 h | 1 Week | 2 Weeks | ||
| Biocoal V1, (350 °C) | 6.05 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Biocoal V2 (impregnated with CaCl2, 350 °C) | 8.35 | ✓ | ✓ | ✓ | |||
| Biocoal V3 (impregnated with MgCl2, 350 °C) | 8.30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Biocoal V5 (impregnated with CaCl2, 600 °C) | 8.30 | ✓ | ✓ | ✓ | |||
| Biocoal V6 (impregnated with MgCl2, 600 °C) | 5.79 | ✓ | ✓ | ✓ | |||
| Commercial biocoal | 9.99 | ✓ | ✓ | ✓ | |||
| Commercial biocoal + MgCl2 | 9.99 + 1.78 | ✓ | ✓ | ✓ | |||
| Parameter | Reactor #1 | Reactor #2 | Secondary Reactor |
|---|---|---|---|
| OLR, in kgoDM∙(m³∙d)−1 | 3.42 | 3.26 | 2.24 * |
| 0.41 ** | |||
| HRT, in d | 61.50 | 58.00 | 32.40 |
| Temperature (mean ± SD), in °C | 44.00 ± 2.90 | 42.20 ± 3.20 | 52.50 ± 5.00 |
| pH | NA | 8.20 ± 0.41 | |
| DM, in %FM | NA | 7.54 ± 0.84 | |
| oDM, g∙kgDM−1 | NA | 681.16 ± 15.23 | |
| NH4, g∙kgDM−1 | NA | 61.58 ± 3.46 | |
| P, g∙kgDM−1 | NA | 13.86± 0.32 | |
| K, g∙kgDM−1 | NA | 83.37 ± 5.35 | |
| Ca, g∙kgDM−1 | NA | 26.78 ± 1.34 | |
| Mg, g∙kgDM−1 | NA | 7.76 ± 0.39 | |
| Variant | C, in% | H, in% | N, in% |
|---|---|---|---|
| Biocoal V1 (350 °C) | 64.32 ± 3.00 | 1.95 ± 0.49 | 0.26 ± 0.02 |
| Biocoal V4 (600 °C) | 77.56 ± 6.07 | 2.08 ± 0.48 | 0.25 ± 0.03 |
| Variant | Bulk Density (Mean ± SD), in kg∙m−3 | Particle Size (Mean ± SD), in mm | DM, (Mean ± SD), in g∙kgFM−1 | oDM, (Mean ± SD), in g∙kgDM−1 | Ca, (Mean ± SD), in g∙kgFM−1 | Mg, (Mean ± SD), in g∙kgFM−1 |
|---|---|---|---|---|---|---|
| Biocoal V1, (350 °C) | 176.55 ± 13.34 | 7.00 ± 3.00 | 1024.61 ± 2.72 | 969.97 ± 2.29 | 3.66 ± 0.58 | 1.53 ± 0.51 |
| Biocoal V2 (impregnated with CaCl2, 350 °C) | 261.43 ± 1.53 | 10.00 ± 5.00 | 1047.02 ± 2.93 | 549.71 ± 3.59 | 165.79 ± 8.73 | 0.52 ± 0.04 |
| Biocoal V3 (impregnated with MgCl2, 350 °C) | 243.55 ± 5.47 | 9.00 ± 3.00 | 1041.02 ± 0.98 | 811.11 ± 2.79 | 3.10 ± 0.19 | 101.16 ± 3.31 |
| Biocoal V4, (600 °C) | 158.19 ± 2.36 | 5.00 ± 2.00 | 1002.23 ± 0.92 | 1006.03 ± 98.63 | 7.95 ± 1.40 | 2.74 ± 0.79 |
| Biocoal V5 (impregnated with CaCl2, 600 °C) | 284.48 ± 0.61 | 7.00 ± 3.00 | 1030.99 ± 0.57 | 482.36 ± 11.06 | 166.11 ± 22.29 | 0.56 ± 0.01 |
| Biocoal V6 (impregnated with MgCl2, 600 °C) | 217.11 ± 1.44 | 7.00 ± 3.00 | 1033.45 ± 2.31 | 489.31 ± 3.41 | 3.67 ± 0.09 | 145.05 ± 4.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, I.; Lemmer, A. Nutrient Recovery from Digestate of Agricultural Biogas Plants: A Comparative Study of Innovative Biocoal-Based Additives in Laboratory and Full-Scale Experiments. Molecules 2022, 27, 5289. https://doi.org/10.3390/molecules27165289
Morozova I, Lemmer A. Nutrient Recovery from Digestate of Agricultural Biogas Plants: A Comparative Study of Innovative Biocoal-Based Additives in Laboratory and Full-Scale Experiments. Molecules. 2022; 27(16):5289. https://doi.org/10.3390/molecules27165289
Chicago/Turabian StyleMorozova, Ievgeniia, and Andreas Lemmer. 2022. "Nutrient Recovery from Digestate of Agricultural Biogas Plants: A Comparative Study of Innovative Biocoal-Based Additives in Laboratory and Full-Scale Experiments" Molecules 27, no. 16: 5289. https://doi.org/10.3390/molecules27165289