Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties
Abstract
:1. Introduction
2. Traditional Uses as Food and Medicine
3. Nutritional and Chemical Composition
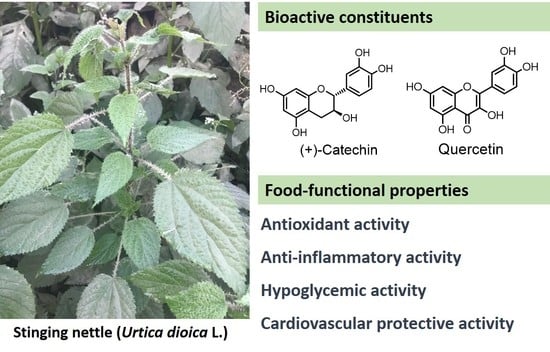
4. Food Functional Activities
4.1. Antioxidant Property
4.2. Anti-Inflammatory Property
4.3. Hypoglycemic Property
4.4. Antiulcer Activities
4.5. Antibacterial Activities
4.6. Cardiovascular-Related Activities
4.7. Activities Related to Brain Disorders
4.8. Activities Related to Allergic Rhinitis
5. Clinical Studies on Urtica dioica
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, R.M.; Bussmann, R.W. Ethnobotany in the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2008, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Karki, R.; Kim, D.W. Cepharanthine Inhibits In Vitro VSMC Proliferation and Migration and Vascular Inflammatory Responses Mediated by RAW264.7. Toxicol. In Vitro 2016, 34, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-Inflammatory and Anticancer Activities of Naringenin-Loaded Liquid Crystalline Nanoparticles In Vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Paudel, K.R.; Jeong, J.; Wi, A.J.; Park, W.S.; Kim, D.W.; Oak, M.H. Antiatherogenic Effect of Camellia Japonica Fruit Extract in High Fat Diet-Fed Rats. Evid. Based Complement. Altern. Med. 2016, 2016, 9679867. [Google Scholar] [CrossRef]
- Paudel, K.R.; Lee, U.W.; Kim, D.W. Chungtaejeon, a Korean Fermented Tea, Prevents the Risk of Atherosclerosis in Rats Fed a High-Fat Atherogenic Diet. J. Integr. Med. 2016, 14, 134–142. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Gong, D.S.; Oak, M.H. Vascular Protection by Ethanol Extract of Morus Alba Root Bark: Endothelium-Dependent Relaxation of Rat Aorta and Decrease of Smooth Muscle Cell Migration and Proliferation. Evid. Based Complement. Altern. Med. eCAM 2018, 2018, 7905763. [Google Scholar] [CrossRef]
- Rehman, A.; Mehmood, M.H.; Haneef, M.; Gilani, A.H.; Ilyas, M.; Siddiqui, B.S.; Ahmed, M. Potential of Black Pepper as a Functional Food for Treatment of Airways Disorders. J. Funct. Foods 2015, 19, 126–140. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Devkota, H.P.; Adhikari-Devkota, A.; Paudel, K.R.; Panth, N.; Chellappan, D.K.; Hansbro, P.M.; Dua, K. Tea (Catechins Including (−)-Epigallocatechin-3-Gallate) and Cancer. In Nutraceuticals and Cancer Signaling. Food Bioactive Ingredients; Jafari, S.M., Nabavi, S.M., Silva, A.S., Eds.; Springer: Cham, Switzerland, 2021; pp. 451–466. [Google Scholar] [CrossRef]
- Gacche, R.N. Functional Foods, Nutraceuticals and Dietary Supplements in Cancer Prevention. In Dietary Research and Cancer; Springer: Singapore, 2021; pp. 121–130. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced Research on the Antioxidant and Health Benefit of Elderberry (Sambucus Nigra) in Food—A Review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- De Vico, G.; Guida, V.; Carella, F. Urtica dioica (Stinging Nettle): A neglected plant with emerging growth promoter/immunostimulant properties for farmed fish. Front. Physiol. 2018, 9, 285. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging Nettle (Urtica dioica L.): A Reservoir of Nutrition and Bioactive Components with Great Functional Potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging Nettle, Urtica dioica L.: Botanical, Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Manandhar, N.P. Plants and People of Nepal; Timber Press, Inc.: Portland, OR, USA, 2002. [Google Scholar]
- Watanabe, T.; Rajbhandari, K.R.; Malla, K.J.; Devkota, H.P.; Yahara, S. A Handbook of Medicinal Plants of Nepal Supplement I; Ayurseed, L.E.I.: Kanagawa, Japan, 2013. [Google Scholar]
- Randall, C.; Meethan, K.; Randall, H.; Dobbs, F. Nettle Sting of Urtica dioica for Joint Pain—An Exploratory Study of This Complementary Therapy. Complement. Ther. Med. 1999, 7, 126–131. [Google Scholar] [CrossRef]
- Randall, C.; Randall, H.; Dobbs, F.; Hutton, C.; Sanders, H. Randomized Controlled Trial of Nettle Sting for Treatment of Base-of-Thumb Pain. J. R. Soc. Med. 2000, 93, 305–309. [Google Scholar] [CrossRef]
- Singh, H. Ethnobotanical Studies on Urtica dioica Linn. among the Bhotias of Chamoli Garhwal, U.P. J. Econ. Taxon. Bot. 1989, 13, 719–724. [Google Scholar]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic Compounds Analysis of Root, Stalk, and Leaves of Nettle. Sci. World J. 2012, 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Uprety, Y.; Poudel, R.C.; Shrestha, K.K.; Rajbhandary, S.; Tiwari, N.N.; Shrestha, U.B.; Asselin, H. Diversity of Use and Local Knowledge of Wild Edible Plant Resources in Nepal. J. Ethnobiol. Ethnomed. 2012, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Upton, R. Stinging Nettles Leaf (Urtica dioica L.): Extraordinary Vegetable Medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1664. [Google Scholar] [CrossRef]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Shaheen, S.; Azmi, L.; Prakash Mishra, A.; Sener, B.; et al. Urtica dioica -Derived Phytochemicals for Pharmacological and Therapeutic Applications. Evid.-Based Complement. Altern. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of Nutritional Properties of Stinging Nettle (Urtica dioica) Flour with Wheat and Barley Flours. Food Sci. Nutr. 2016, 4, 119–124. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the Alimurgic Plants Taraxacum Officinale, Papaver Rhoeas and Urtica dioica by Combined NMR and GC–MS Analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef]
- Gül, S.; Emirci, B.; Başer, K.H.; Akpulat, H.A.; Aksu, P. Chemical Composition and in Vitro Cytotoxic, Genotoxic Effects of Essential Oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012, 88, 666–671. [Google Scholar] [CrossRef]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Pertonijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical Composition of Stinging Nettle Leaves Obtained by Different Analytical Approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Orcic, D.; Franciškovic, M.; Bekvalac, K.; Svircev, E.; Beara, I.; Lesjak, M.; Mimica-Dukic, N. Quantitative Determination of Plant Phenolics in Urtica dioica Extracts by High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometric Detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Torija Isasa, M.E. Fatty Acids and Carotenoids from Stinging Nettle (Urtica dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Güder, A.; Korkmaz, H. Evaluation of In-Vitro Antioxidant Properties of Hydroalcoholic Solution Extracts Urtica dioica L., Malva Neglecta Wallr. and Their Mixture. Iran. J. Pharm. Res. 2012, 11, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic Composition and Antioxidant Properties of Some Traditionally Used Medicinal Plants Affected by the Extraction Time and Hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Kushwaha, P.; Verma, S.; Gupta, A.; Srivastava, S.; Rawat, A. Pharmacognostic Evaluation and Antioxidant Activity of Urtica dioica L. Chin. Med. 2012, 3, 128–135. [Google Scholar] [CrossRef]
- Sarma Kataki, M. Antioxidant, Hepatoprotective, and Anthelmintic Activities of Methanol Extract of Urtica dioica L. Leaves. Pharm. Crops 2012, 3, 38–46. [Google Scholar] [CrossRef]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.R.; Seigneuret, J.M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. L’ortie (Urtica dioica L.), Une Source de Produits Antioxidants et Phytochimiques Anti-Âge Pour Des Applications En Cosmétique. Comptes Rendus Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Ahmadipour, B.; Khajali, F. Expression of Antioxidant Genes in Broiler Chickens Fed Nettle ( Urtica dioica) and Its Link with Pulmonary Hypertension. Anim. Nutr. 2019, 5, 264–269. [Google Scholar] [CrossRef]
- Rahmati, M.; Keshvari, M.; Mirnasouri, R.; Chehelcheraghi, F. Exercise and Urtica dioica Extract Ameliorate Hippocampal Insulin Signaling, Oxidative Stress, Neuroinflammation, and Cognitive Function in STZ-Induced Diabetic Rats. Biomed. Pharmacother. 2021, 139, 111577. [Google Scholar] [CrossRef]
- Roschek, B.; Fink, R.C.; McMichael, M.; Alberte, R.S. Nettle Extract (Urtica dioica) Affects Key Receptors and Enzymes Associated with Allergic Rhinitis. Phytother. Res. 2009, 23, 920–926. [Google Scholar] [CrossRef]
- Johnson, T.A.; Sohn, J.; Inman, W.D.; Bjeldanes, L.F.; Rayburn, K. Lipophilic Stinging Nettle Extracts Possess Potent Anti-Inflammatory Activity, Are Not Cytotoxic and May Be Superior to Traditional Tinctures for Treating Inflammatory Disorders. Phytomedicine 2013, 20, 143–147. [Google Scholar] [CrossRef]
- Obertreis, B.; Ruttkowski, T.; Teucher, T.; Behnke, B.; Schmitz, H. Ex-Vivo in-Vitro Inhibition of Lipopolysaccharide Stimulated Tumor Necrosis Factor-α and Interleukin-1β Secretion in Human Whole Blood by Extractum Urticae Dioicae Foliorum. Arzneim. Forsch. Drug Res. 1996, 46, 389–394. [Google Scholar]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.H.; Bhat, T.M.; Sharma, P. Pharmacological and Toxicological Evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Mohammadian, M.; Dianat, M. Antidiabetic Effect of Hydroalcholic Urtica dioica Leaf Extract in Male Rats with Fructose-Induced Insulin Resistance. Iran. J. Med. Sci. 2012, 37, 181–186. [Google Scholar] [PubMed]
- Golalipour, M.J.; Khori, V. The Protective Activity of Urtica dioica Leaves on Blood Glucose Concentration and β-Cells in Streptozotocin-Diabetic Rats. Pak. J. Biol. Sci. 2007, 10, 1200–1204. [Google Scholar] [CrossRef]
- Bnouham, M.; Merhfour, F.Z.; Ziyyat, A.; Mekhfi, H.; Aziz, M.; Legssyer, A. Antihyperglycemic Activity of the Aqueous Extract of Urtica dioica. Fitoterapia 2003, 74, 677–681. [Google Scholar] [CrossRef]
- Ranjbari, A.; Azarbayjani, M.A.; Yusof, A.; Mokhtar, A.H.; Akbarzadeh, S.; Ibrahim, M.Y.; Tarverdizadeh, B.; Farzadinia, P.; Hajiaghaee, R.; Dehghan, F. In Vivo and In Vitro Evaluation of the Effects of Urtica dioica and Swimming Activity on Diabetic Factors and Pancreatic Beta Cells. BMC Complement. Altern. Med. 2016, 16, 101. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioǧlu, Ö.I.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Burkova, V.N.; Boev, S.G.; Vengerovsky, A.I.; Yudina, N.V.; Arbuzov, A.G. Gastroprotective Action of Nettle Extract in Experimental Peptic Ulcer. Eksperimental’naya I Klin. Farmakol. 2011, 74, 24–27. [Google Scholar] [CrossRef]
- Ghaima, K.K.; Hashim, N.M.; Ali, S.A. Antibacterial and Antioxidant Activities of Ethyl Acetate Extract of Nettle (Urtica dioica) and Dandelion (Taraxacum Officinale). J. Appl. Pharm. Sci. 2013, 3, 96–99. [Google Scholar] [CrossRef]
- Modarresi-Chahardehi, A.; Ibrahim, D.; Sulaiman, S.F.; Mousavi, L. Screening Antimicrobial Activity of Various Extracts of Urtica dioica. Rev. Biol. Trop. 2012, 60, 1567–1576. [Google Scholar] [CrossRef]
- Zenão, S.; Aires, A.; Dias, C.; Saavedra, M.J.; Fernandes, C. Antibacterial Potential of Urtica dioica and Lavandula Angustifolia Extracts against Methicillin Resistant Staphylococcus Aureus Isolated from Diabetic Foot Ulcers. J. Herb. Med. 2017, 10, 53–58. [Google Scholar] [CrossRef]
- Testai, L.; Chericoni, S.; Calderone, V.; Nencioni, G.; Nieri, P.; Morelli, I.; Martinotti, E. Cardiovascular Effects of Urtica dioica L. (Urticaceae) Roots Extracts: In Vitro and In Vivo Pharmacological Studies. J. Ethnopharmacol. 2002, 81, 105–109. [Google Scholar] [CrossRef]
- Tahri, A.; Yamani, S.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M.; Ziyyat, A. Acute Diuretic, Natriuretic and Hypotensive Effects of a Continuous Perfusion of Aqueous Extract of Urtica dioica in the Rat. J. Ethnopharmacol. 2000, 73, 95–100. [Google Scholar] [CrossRef]
- Qayyum, R.; Qamar, H.M.-U.D.; Khan, S.; Salma, U.; Khan, T.; Shah, A.J. Mechanisms Underlying the Antihypertensive Properties of Urtica dioica. J. Transl. Med. 2016, 14, 254. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Canter, R.G.; Penney, J.; Tsai, L.H. The Road to Restoring Neural Circuits for the Treatment of Alzheimer’s Disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Abeliovich, A.; Gitler, A.D. Defects in Trafficking Bridge Parkinson’s Disease Pathology and Genetics. Nature 2016, 539, 207–216. [Google Scholar] [CrossRef]
- Patel, S.S.; Mahindroo, N.; Udayabanu, M. Urtica dioica Leaves Modulates Hippocampal Smoothened-Glioma Associated Oncogene-1 Pathway and Cognitive Dysfunction in Chronically Stressed Mice. Biomed. Pharmacother. 2016, 83, 676–686. [Google Scholar] [CrossRef]
- Bisht, R.; Joshi, B.C.; Kalia, A.N.; Prakash, A. Antioxidant-Rich Fraction of Urtica dioica Mediated Rescue of Striatal Mito-Oxidative Damage in MPTP-Induced Behavioral, Cellular, and Neurochemical Alterations in Rats. Mol. Neurobiol. 2017, 54, 5632–5645. [Google Scholar] [CrossRef]
- Toldy, A.; Stadler, K.; Sasvári, M.; Jakus, J.; Jung, K.J.; Chung, H.Y.; Berkes, I.; Nyakas, C.; Radák, Z. The Effect of Exercise and Nettle Supplementation on Oxidative Stress Markers in the Rat Brain. Brain Res. Bull. 2005, 65, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Toldy, A.; Atalay, M.; Stadler, K.; Sasvári, M.; Jakus, J.; Jung, K.J.; Chung, H.Y.; Nyakas, C.; Radák, Z. The Beneficial Effects of Nettle Supplementation and Exercise on Brain Lesion and Memory in Rat. J. Nutr. Biochem. 2009, 20, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Moradzadeh, M.; Hosseini, M.; Beheshti, F.; Sadeghnia, H.R. Beneficial Effects of Urtica dioica on Scopolamine-Induced Memory Impairment in Rats: Protection against Acetylcholinesterase Activity and Neuronal Oxidative Damage. Drug Chem. Toxicol. 2019, 42, 167–175. [Google Scholar] [CrossRef]
- FitzGerald, G.A. COX-2 and beyond: Approaches to Prostaglandin Inhibition in Human Disease. Nat. Rev. Drug Discov. 2003, 2, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Nantel, F.; Fong, C.; Lamontagne, S.; Wright, D.H.; Giaid, A.; Desrosiers, M.; Metters, K.M.; O’Neill, G.P.; Gervais, F.G. Expression of Prostaglandin D Synthase and the Prostaglandin D2 Receptors DP and CRTH2 in Human Nasal Mucosa. Prostaglandins Other Lipid Mediat. 2004, 73, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Bakhshaee, M.; Mohammad pour, A.H.; Esmaeili, M.; Azad, F.J.; Talesh, G.A.; Salehi, M.; Mohajer, M.N. Efficacy of Supportive Therapy of Allergic Rhinitis by Stinging Nettle (Urtica dioica) Root Extract: A Randomized, Double-Blind, Placebo- Controlled, Clinical Trial. Iran. J. Pharm. Res. IJPR 2017, 16, 112. [Google Scholar] [CrossRef]
- Kianbakht, S.; Khalighi-Sigaroodi, F.; Dabaghian, F.H. Improved Glycemic Control in Patients with Advanced Type 2 Diabetes Mellitus Taking Urtica dioica Leaf Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Clin. Lab. 2013, 59, 1071–1076. [Google Scholar] [CrossRef]
- Khalili, N.; Fereydoonzadeh, R.; Mohtashami, R.; Mehrzadi, S.; Heydari, M.; Huseini, H.F. Silymarin, Olibanum, and Nettle, A Mixed Herbal Formulation in the Treatment of Type II Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. J. Evid. Based Complement. Altern. Med. 2017, 22, 603. [Google Scholar] [CrossRef]
- Moré, M.; Gruenwald, J.; Pohl, U.; Uebelhack, R. A Rosa Canina—Urtica dioica—Harpagophytum Procumbens/Zeyheri Combination Significantly Reduces Gonarthritis Symptoms in a Randomized, Placebo-Controlled Double-Blind Study. Planta Med. 2017, 83, 1384–1391. [Google Scholar] [CrossRef]
- Karami, A.A.; Sheikhsoleimani, M.; Memarzadeh, M.R.; Haddadi, E.; Bakhshpour, M.; Mohammadi, N.; Mirhashemi, S.M. Urtica dioica Root Extract on Clinical and Biochemical Parameters in Patients with Benign Prostatic Hyperplasia, Randomized Controlled Trial. Pak. J. Biol. Sci. 2020, 23, 1338–1344. [Google Scholar] [CrossRef]



| Chemical Group | Compounds | References |
|---|---|---|
| Flavonoids | Amentoflavone, apiin, apigenin, apigenin 7-O-β-d-glucoside, baicalin, baicalein, catechin, epicatechin, epigallocatechin gallate, chrysoeriol, genestein, isorhamnetin, kaempferol, keampferol 3-O-β-d-glucoside, luteolin, luteolin 7-O-β-d-glucoside, myrecetin, naringenin, quercetin, quercetin 3-O-β-d-glucoside, quercetin 3-O-β-d-galactoside, rutin, vitexin | [25,34,35] |
| Phenolic acids | Hydroxybenzoic acid derivatives Gallic acid, vanillic acid, syringic acid, protocatechuic acid, gentisic acid Cinnamic acid derivatives Cinnamic acid, caffeic acid, p-coumaric acid, ferulic acid, chlorogenic acid, sinapic acid | [25,34,35] |
| Amino acids | Alanine, γ-aminobutyric acid (GABA), glutamic acid, isoleucine, leucine, phenylalanine, proline, tyrosine, valine | [32] |
| Carotenoids | β-Carotene, lutein isomers, neoxanthin, violaxanthin | [36] |
| Organic acids | Acetic acid, citric acid, formic acid, malic acid, succinic acid | [32] |
| Fatty acids | Arachidic acid, arachidonic acid, behenic acid, dodecendioic acid, euric acid, palmitic acid, palmitolic acid, stearic acid, tricosanoic acid, lauric acid, etc. | [34,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. https://doi.org/10.3390/molecules27165219
Devkota HP, Paudel KR, Khanal S, Baral A, Panth N, Adhikari-Devkota A, Jha NK, Das N, Singh SK, Chellappan DK, et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules. 2022; 27(16):5219. https://doi.org/10.3390/molecules27165219
Chicago/Turabian StyleDevkota, Hari Prasad, Keshav Raj Paudel, Shristi Khanal, Ananda Baral, Nisha Panth, Anjana Adhikari-Devkota, Niraj Kumar Jha, Niranjan Das, Sachin Kumar Singh, Dinesh Kumar Chellappan, and et al. 2022. "Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties" Molecules 27, no. 16: 5219. https://doi.org/10.3390/molecules27165219