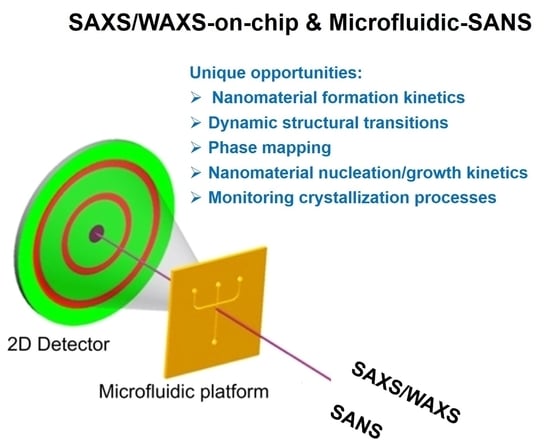
Microfluidic Nanomaterial Synthesis and In Situ SAXS, WAXS, or SANS Characterization: Manipulation of Size Characteristics and Online Elucidation of Dynamic Structural Transitions
Abstract
:1. Introduction
2. Two-Dimensional and Three-Dimensional Specialized and Compatible Microfluidic Chips for In Situ Characterization Studies
| Microfluidics | Features, Advantages, and Reported Main Drawbacks | Characterization and Phase Mapping | Ref. |
|---|---|---|---|
| 2D HFF platform based on thiol-ene | Suitability for X-ray studies; lipid adsorption prevention; and disposability. Main drawback: low efficacy in controlling the nanoparticle size characteristics. |
| [53] [31] [30] |
| Different polyimide- based chips | Good resistance to X-ray; suitability for SAXS-scanning studies: investigation of orientation and structural features of self-assemblies; thermal stability; and compatibility to organic solvents. |
| [34] [74] |
| Cyclic olefin copolymer (COC) devices | Prevention of leakage (fabrication from COCs only, no need for gluing between interfaces); suitability for X-ray studies; and high X-ray transmission and radiation resistance. Main drawbacks: incompatibility with tetrahydrofuran and instability at relatively high temperatures [49]. |
| [73] |
| Laser lithography (LL) chips | High transparency and low X-ray background scattering; and suitability for X-ray studies. |
| [72] |
| 3D polyimide chips | A more efficient and uniform mixing as compared to 2D polyimide chips; a combination of suitability for X-ray studies and compatibility to organic solvents with 3D focusing. The employed laser micromachining procedure is also reliable. |
| [49] |
| Platform based on thiol-ene | Pressure and temperature resistance; capability to handle viscous fluids; suitability for X-ray studies. Interesting features: SAXS set-up allows controlling the temperature and conducting SAXS experiments at relatively high temperature. |
| [71] |
| A custom-built crown glass contraction– expansion device | Suitability for SANS studies; enabling tubular flow in continuous and oscillatory modes, relevance to industrial continuous and tubular flow processes. |
| [51] |
| 3D Kapton- based flow-focusing device | Compatibility to organic solvents; suitability for SAXS studies; a high spatial and temporal resolution; and rapid and efficient mixing of solvents. |
| [43] |
| Droplet-based device | Suitability for X-ray studies; a high-throughput analysis; automatic screening of variable crystallization conditions. |
| [45] |
| Silicon/glass chips | High cost; suitability for SAXS and SANS studies; compatibility to organic solvents; and a time-consuming fabrication. |
| [47] |
| Epoxy-based chips | Good conditions for SAXS studies; X-ray and optical transparency; pressure resistance up to 2.9 bar; chemical resistance to certain solvents. Main drawbacks: incompatibility to some organic solvents, including tetrahydrofuran. |
| [70] |
| Thiol-ene- epoxy (OSTE+) droplet devices | Suitability for SAXS/WAXS studies; X-ray transparency; X-ray signal quality of OSTE+ material as compared to typically used polyimide (Kapton). |
| [57] |
3. Continuous Production and In Situ Characterization of Nano-Self-Assemblies Attractive for Drug or Functional Food Delivery, or Vaccine Development
4. In Situ Phase Behavior and Structural Dynamics Investigations of Amphiphilic Polymers and Lipids
5. In Situ Structural Dynamics and Kinetic Formation Investigations of Hard Solid Nanoparticles
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T.F. Production of nanoparticle drug delivery systems with microfluidics tools. Expert Opin. Drug Deliv. 2015, 12, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Yaldiz, B.; Bor, G.; Yaghmur, A.; Yesil-Celiktas, O. Advances in microfluidic synthesis and coupling with synchrotron SAXS for continuous production and real-time structural characterization of nano-self-assemblies. Colloid Surf. B 2021, 201, 111633. [Google Scholar] [CrossRef]
- Mao, K.; Min, X.; Zhang, H.; Zhang, K.; Cao, H.; Guo, Y.; Yang, Z. Paper-based microfluidics for rapid diagnostics and drug delivery. J. Control. Release 2020, 322, 187–199. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic devices for drug delivery systems and drug screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Briones, J.; Espulgar, W.; Koyama, S.; Takamatsu, H.; Tamiya, E.; Saito, M. The future of microfluidics in immune checkpoint blockade. Cancer Gene Ther. 2021, 28, 895–910. [Google Scholar] [CrossRef]
- Del Giudice, F. A Review of Microfluidic Devices for Rheological Characterisation. Micromachines 2022, 13, 167. [Google Scholar] [CrossRef]
- Xiong, Q.; Lim, C.Y.; Ren, J.; Zhou, J.; Pu, K.; Chan-Park, M.B.; Mao, H.; Lam, Y.C.; Duan, H. Magnetic nanochain integrated microfluidic biochips. Nat. Commun. 2018, 9, 1743. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.H.; Shin, H.H.; Choi, H.W.; Lim, J.H.; Mo, S.J.; Ahrberg, C.D.; Lee, J.M.; Chung, B.G. Electro-responsive hydrogel-based microfluidic actuator platform for photothermal therapy. Lab Chip 2020, 20, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.; Jeon, N.L. Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; Devoe, D.L.; Gaitan, M. Microfluidic Mixing and the Formation of Nanoscale Lipid Vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Microfluidic directed formation of liposomes of controlled size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef]
- Firmino, P.C.; Vianna, S.S.; da Costa, O.M.; Malfatti-Gasperini, A.A.; Gobbi, A.L.; Lima, R.S.; Lucimara, G. 3D micromixer for nanoliposome synthesis: A promising advance in high mass productivity. Lab Chip 2021, 21, 2971–2985. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Ghazal, A.; Sartori, B.; Dimaki, M.; Svendsen, W.E.; Yesil-Celiktas, O.; Yaghmur, A. Continuous Microfluidic Production of Citrem-Phosphatidylcholine Nano-Self-Assemblies for Thymoquinone Delivery. Nanomaterials 2021, 11, 1510. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Chang, Y.; Alfeche, A.; Leal, C. Microfluidics Synthesis of Gene Silencing Cubosomes. ACS Nano 2018, 12, 9196–9205. [Google Scholar] [CrossRef]
- Yaghmur, A.; Ghazal, A.; Ghazal, R.; Dimaki, M.; Svendsen, W.E. A hydrodynamic flow focusing microfluidic device for the continuous production of hexosomes based on docosahexaenoic acid monoglyceride. Phys. Chem. Chem. Phys. 2019, 21, 13005–13013. [Google Scholar] [CrossRef]
- Sebastian, V. Toward continuous production of high-quality nanomaterials using microfluidics: Nanoengineering the shape, structure and chemical composition. Nanoscale 2022, 14, 4411–4447. [Google Scholar] [CrossRef]
- Pilkington, C.P.; Seddon, J.M.; Elani, Y. Microfluidic technologies for the synthesis and manipulation of biomimetic membranous nano-assemblies. Phys. Chem. Chem. Phys. 2021, 23, 3693–3706. [Google Scholar] [CrossRef]
- Streck, S.; Hong, L.; Boyd, B.J.; McDowell, A. Microfluidics for the production of nanomedicines: Considerations for polymer and lipid-based systems. Pharm. Nanotechnol. 2019, 7, 423–443. [Google Scholar] [CrossRef]
- Huang, P.H.; Zhao, S.; Bachman, H.; Nama, N.; Li, Z.; Chen, C.; Yang, S.; Wu, M.; Zhang, S.P.; Huang, T.J. Acoustofluidic synthesis of particulate nanomaterials. Adv. Sci. 2019, 6, 1900913. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 2106580, in press. [Google Scholar] [CrossRef]
- Tian, F.; Cai, L.; Liu, C.; Sun, J. Microfluidic technologies for nanoparticle formation. Lab Chip 2022, 22, 512–529. [Google Scholar] [CrossRef]
- Prakash, G.; Shokr, A.; Willemen, N.; Bashir, S.M.; Shin, S.R.; Hassan, S. Microfluidic fabrication of lipid nanoparticles for the delivery of nucleic acids. Adv. Drug Deliv. Rev. 2022, 184, 114197. [Google Scholar] [CrossRef]
- Hood, R.; Vreeland, W.; DeVoe, D.L. Microfluidic remote loading for rapid single-step liposomal drug preparation. Lab Chip 2014, 14, 3359–3367. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.F.B. SAXS on a chip: From dynamics of phase transitions to alignment phenomena at interfaces studied with microfluidic devices. Phys. Chem. Chem. Phys. 2017, 19, 23690–23703. [Google Scholar] [CrossRef]
- Martin, H.P.; Brooks, N.J.; Seddon, J.M.; Luckham, P.F.; Terrill, N.J.; Kowalski, A.J.; Cabral, J.T. Microfluidic processing of concentrated surfactant mixtures: Online SAXS, microscopy and rheology. Soft Matter 2016, 12, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Khaliqi, K.; Ghazal, A.; Azmi, I.D.M.; Amenitsch, H.; Mortensen, K.; Salentinig, S.; Yaghmur, A. Direct monitoring of lipid transfer on exposure of citrem nanoparticles to an ethanol solution containing soybean phospholipids by combining synchrotron SAXS with microfluidics. Analyst 2017, 142, 3118–3126. [Google Scholar] [CrossRef]
- Ghazal, A.; Gontsarik, M.; Kutter, J.P.; Lafleur, J.P.; Ahmadvand, D.; Labrador, A.; Salentinig, S.; Yaghmur, A. Microfluidic Platform for the Continuous Production and Characterization of Multilamellar Vesicles: A Synchrotron Small-Angle X-ray Scattering (SAXS) Study. J. Phys. Chem. Lett. 2017, 8, 73–79. [Google Scholar] [CrossRef]
- Silva, B.F.B.; Zepeda-Rosales, M.; Venkateswaran, N.; Fletcher, B.J.; Carter, L.G.; Matsui, T.; Weiss, T.M.; Han, J.; Li, Y.L.; Olsson, U.; et al. Nematic Director Reorientation at Solid and Liquid Interfaces under Flow: SAXS Studies in a Microfluidic Device. Langmuir 2015, 31, 4361–4371. [Google Scholar] [CrossRef]
- Schwemmer, F.; Blanchet, C.E.; Spilotros, A.; Kosse, D.; Zehnle, S.; Mertens, H.D.T.; Graewert, M.A.; Rossle, M.; Paust, N.; Svergun, D.I.; et al. LabDisk for SAXS: A centrifugal microfluidic sample preparation platform for small-angle X-ray scattering. Lab Chip 2016, 16, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Lutz-Bueno, V.; Zhao, J.G.; Mezzenga, R.; Pfohl, T.; Fischer, P.; Liebi, M. Scanning-SAXS of microfluidic flows: Nanostructural mapping of soft matter. Lab Chip 2016, 16, 4028–4035. [Google Scholar] [CrossRef]
- Buscema, M.; Deyhle, H.; Pfohl, T.; Zumbuehl, A.; Müller, B. Spatially resolved small-angle X-ray scattering for characterizing mechanoresponsive liposomes using microfluidics. Mater. Today Bio 2019, 1, 100003. [Google Scholar] [CrossRef]
- Haider, R.; Marmiroli, B.; Gavalas, I.; Wolf, M.; Matteucci, M.; Taboryski, R.; Boisen, A.; Stratakis, E.; Amenitsch, H. Laser ablation and injection moulding as techniques for producing micro channels compatible with Small Angle X-Ray Scattering. Microelectron. Eng. 2018, 195, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.G.; Watanabe, T.; Adamo, M.; Martel, A.; Porcar, L.; Cabral, J.T. Microfluidic devices for small-angle neutron scattering. J. Appl. Cryst. 2018, 51, 570–583. [Google Scholar] [CrossRef]
- Hertzog, D.E.; Michalet, X.; Jäger, M.; Kong, X.; Santiago, J.G.; Weiss, S.; Bakajin, O. Femtomole mixer for microsecond kinetic studies of protein folding. Anal. Chem. 2004, 76, 7169–7178. [Google Scholar] [CrossRef] [Green Version]
- Polte, J.; Erler, R.; Thunemann, A.F.; Sokolov, S.; Ahner, T.T.; Rademann, K.; Emmerling, F.; Kraehnert, R. Nucleation and growth of gold nanoparticles studied via in situ small angle X-ray scattering at millisecond time resolution. ACS Nano 2010, 4, 1076–1082. [Google Scholar] [CrossRef]
- Lignos, I.; Maceiczyk, R.; deMello, A.J. Microfluidic technology: Uncovering the mechanisms of nanocrystal nucleation and growth. Acc. Chem. Res. 2017, 50, 1248–1257. [Google Scholar] [CrossRef]
- Herbst, M.; Hofmann, E.; Förster, S. Nucleation and growth kinetics of ZnO nanoparticles studied by in situ microfluidic SAXS/WAXS/UV–Vis experiments. Langmuir 2019, 35, 11702–11709. [Google Scholar] [CrossRef] [PubMed]
- Radajewski, D.; Hunter, L.; He, X.; Nahi, O.; Galloway, J.M.; Meldrum, F.C. An innovative data processing method for studying nanoparticle formation in droplet microfluidics using X-rays scattering. Lab Chip 2021, 21, 4498–4506. [Google Scholar] [CrossRef]
- Merkens, S.; Vakili, M.; Sánchez-Iglesias, A.; Litti, L.; Gao, Y.; Gwozdz, P.V.; Sharpnack, L.; Blick, R.H.; Liz-Marzán, L.M.; Grzelczak, M. Time-resolved analysis of the structural dynamics of assembling gold nanoparticles. ACS Nano 2019, 13, 6596–6604. [Google Scholar] [CrossRef] [PubMed]
- Brennich, M.E.; Nolting, J.-F.; Dammann, C.; Nöding, B.; Bauch, S.; Herrmann, H.; Pfohl, T.; Köster, S. Dynamics of intermediate filament assembly followed in micro-flow by small angle X-ray scattering. Lab Chip 2011, 11, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.; Radajewski, D.; Round, A.; Brennich, M.; Pernot, P.; Biscans, B.; Bonneté, F.; Teychené, S. Coupling high throughput microfluidics and small-angle X-ray scattering to study protein crystallization from solution. Anal. Chem. 2017, 89, 2282–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, H.P.; Brooks, N.J.; Seddon, J.M.; Terrill, N.J.; Luckham, P.F.; Kowalski, A.J.; Cabral, J.T. Complex fluids under microflow probed by SAXS: Rapid microfabrication and analysis. J. Phys. Conf. Ser. 2010, 247, 012050. [Google Scholar] [CrossRef]
- Rolley, N.; Bonnin, M.; Lefebvre, G.; Verron, S.; Bargiel, S.; Robert, L.; Riou, J.; Simonsson, C.; Bizien, T.; Gimel, J.-C. Galenic Lab-on-a-Chip concept for lipid nanocapsules production. Nanoscale 2021, 13, 11899–11912. [Google Scholar] [CrossRef]
- Khvostichenko, D.S.; Kondrashkina, E.; Perry, S.L.; Pawate, A.S.; Brister, K.; Kenis, P.J. An X-ray transparent microfluidic platform for screening of the phase behavior of lipidic mesophases. Analyst 2013, 138, 5384–5395. [Google Scholar] [CrossRef] [Green Version]
- Vakili, M.; Merkens, S.; Gao, Y.; Gwozdz, P.V.; Vasireddi, R.; Sharpnack, L.; Meyer, A.; Blick, R.H.; Trebbin, M. 3D micromachined polyimide mixing devices for in situ X-ray imaging of solution-based block copolymer phase transitions. Langmuir 2019, 35, 10435–10445. [Google Scholar] [CrossRef]
- With, S.; Trebbin, M.; Bartz, C.B.; Neuber, C.; Dulle, M.; Yu, S.; Roth, S.V.; Schmidt, H.-W.; Fӧrster, S. Fast diffusion-limited lyotropic phase transitions studied in situ using continuous flow microfluidics/microfocus-SAXS. Langmuir 2014, 30, 12494–12502. [Google Scholar] [CrossRef]
- Donina, L.; Rafique, A.; Khodaparast, S.; Porcar, L.; Cabral, J.T. Lamellar-to-MLV transformation in SDS/octanol/brine examined by microfluidic-SANS and polarised microscopy. Soft Matter 2021, 17, 10053–10062. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Sesen, M.; Hawley, A.; Neild, A.; Spicer, P.T.; Boyd, B.J. Comparison of bulk and microfluidic methods to monitor the phase behaviour of nanoparticles during digestion of lipid-based drug formulations using in situ X-ray scattering. Soft Matter 2019, 15, 9565–9578. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, A.; Gontsarik, M.; Kutter, J.P.; Lafleur, J.P.; Labrador, A.; Mortensen, K.; Yaghmur, A. Direct monitoring of calcium-triggered phase transitions in cubosomes using small-angle X-ray scattering combined with microfluidics. J. Appl. Cryst. 2016, 49, 2005–2014. [Google Scholar] [CrossRef] [Green Version]
- Komorowski, K.; Schaeper, J.; Sztucki, M.; Sharpnack, L.; Brehm, G.; Köster, S.; Salditt, T. Vesicle adhesion in the electrostatic strong-coupling regime studied by time-resolved small-angle X-ray scattering. Soft Matter 2020, 16, 4142–4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Fan, S.; Geng, L.; Lin, J.; Yao, X.; Zhang, Y. Flow analysis of regenerated silk fibroin/cellulose nanofiber suspensions via a bioinspired microfluidic chip. Adv. Mater. Technol. 2021, 6, 2100124. [Google Scholar] [CrossRef]
- Garcia-Lojo, D.; Modin, E.; Gomez-Graña, S.; Imperor-Clerc, M.; Chuvilin, A.; Pastoriza-Santos, I.; Perez-Juste, J.; Constantin, D.; Hamon, C. Structure and formation kinetics of millimeter-size single domain supercrystals. Adv. Funct. Mater. 2021, 31, 2101869. [Google Scholar] [CrossRef]
- Lange, T.; Charton, S.; Bizien, T.; Testard, F.; Malloggi, F. OSTE+ for in situ SAXS analysis with droplet microfluidic devices. Lab Chip 2020, 20, 2990–3000. [Google Scholar] [CrossRef]
- Anaraki, N.I.; Sadeghpour, A.; Iranshahi, K.; Toncelli, C.; Cendrowska, U.; Stellacci, F.; Dommann, A.; Wick, P.; Neels, A. New approach for time-resolved and dynamic investigations on nanoparticles agglomeration. Nano Res. 2020, 13, 2847–2856. [Google Scholar] [CrossRef]
- Narayanan, T.; Konovalov, O. Synchrotron scattering methods for nanomaterials and soft matter research. Materials 2020, 13, 752. [Google Scholar] [CrossRef] [Green Version]
- Yaghmur, A.; Paasonen, L.; Yliperttula, M.; Urtti, A.; Rappolt, M. Structural elucidation of light activated vesicles. J. Phys. Chem. Lett. 2010, 1, 962–966. [Google Scholar] [CrossRef]
- Yaghmur, A.; Sartori, B.; Rappolt, M. The role of calcium in membrane condensation and spontaneous curvature variations in model lipidic systems. Phys. Chem. Chem. Phys. 2011, 13, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Rappolt, M. Structural characterization of lipidic systems under nonequilibrium conditions. Eur. Biophys. J. 2012, 41, 831–840. [Google Scholar] [CrossRef]
- Paasonen, L.; Sipila, T.; Subrizi, A.; Laurinmaki, P.; Butcher, S.J.; Rappolt, M.; Yaghmur, A.; Urtti, A.; Yliperttula, M. Gold-embedded photosensitive liposomes for drug delivery: Triggering mechanism and intracellular release. J. Control. Release 2010, 147, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Grillo, I. Applications of stopped-flow in SAXS and SANS. Curr. Opin. Colloid Interface Sci. 2009, 14, 402–408. [Google Scholar] [CrossRef]
- Yaghmur, A.; Laggner, P.; Sartori, B.; Rappolt, M. Calcium triggered L alpha-H2 phase transition monitored by combined rapid mixing and time-resolved synchrotron SAXS. PLoS ONE 2008, 3, e2072. [Google Scholar] [CrossRef]
- Jensen, G.V.; Lund, R.; Gummel, J.; Monkenbusch, M.; Narayanan, T.; Pedersen, J.S. Direct observation of the formation of surfactant micelles under nonisothermal conditions by synchrotron SAXS. J. Am. Chem. Soc. 2013, 135, 7214–7222. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Drechsler, M.; Lesieur, S. Rapid mixing stopped-flow small-angle X-ray scattering study of lipoplex formation at beamline ID02@ ESRF. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 105–110. [Google Scholar] [CrossRef]
- Yaghmur, A.; Kriechbaum, M.; Amenitsch, H.; Steinhart, M.; Laggner, P.; Rappolt, M. Effects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein-oil systems. Langmuir 2010, 26, 1177–1185. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Yaghmur, A.; Steinhart, M.; Kriechbaum, M.; Rappolt, M. Effects of high pressure on internally self-assembled lipid nanoparticles: A synchrotron small-angle X-ray scattering (SAXS) study. Langmuir 2016, 32, 11907–11917. [Google Scholar] [CrossRef] [Green Version]
- Raffy, S.; Palleau, E.; Calvignac, B.; Brotons, G.; Lefebvre, G.; Rolley, N.; Teychene, S.; Viguier, B.; Cerezo, S.C.; Truan, G. “All in One” Epoxy-Based Microfluidic Chips at Your Fingertips. ACS Appl. Polym. Mater. 2021, 3, 801–810. [Google Scholar] [CrossRef]
- Poulos, A.S.; Nania, M.; Lapham, P.; Miller, R.M.; Smith, A.J.; Tantawy, H.; Caragay, J.; Gummel, J.; Ces, O.; Robles, E.S.J.; et al. Microfluidic SAXS Study of Lamellar and Multilamellar Vesicle Phases of Linear Sodium Alkylbenzenesulfonate Surfactant with Intrinsic Isomeric Distribution. Langmuir 2016, 32, 5852–5861. [Google Scholar] [CrossRef] [Green Version]
- Trantidou, T.; Friddin, M.S.; Gan, K.B.; Han, L.Y.; Bolognesi, G.; Brooks, N.J.; Ces, O. Mask-Free Laser Lithography for Rapid and Low-Cost Microfluidic Device Fabrication. Anal. Chem. 2018, 90, 13915–13921. [Google Scholar] [CrossRef]
- Denz, M.; Brehm, G.; Hémonnot, C.Y.; Spears, H.; Wittmeier, A.; Cassini, C.; Saldanha, O.; Perego, E.; Diaz, A.; Burghammer, M. Cyclic olefin copolymer as an X-ray compatible material for microfluidic devices. Lab Chip 2018, 18, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Palomo, A.; Lutz-Bueno, V.; Cao, X.; Kadar, R.; Andersson, M.; Liebi, M. In Situ Visualization of the Structural Evolution and Alignment of Lyotropic Liquid Crystals in Confined Flow. Small 2021, 17, 2006229. [Google Scholar] [CrossRef]
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1. [Google Scholar] [CrossRef]
- Azmi, I.D.; Wibroe, P.P.; Wu, L.P.; Kazem, A.I.; Amenitsch, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. J. Control. Release 2016, 239, 1–9. [Google Scholar] [CrossRef]
- Shearman, G.C.; Ces, O.; Templer, R.H.; Seddon, J.M. Inverse lyotropic phases of lipids and membrane curvature. J. Phys. Condens. Matter 2006, 18, S1105–S1124. [Google Scholar] [CrossRef]
- Lasic, D.D. Spontaneous vesiculation and spontaneous liposomes. J. Liposome Res. 1999, 9, 43–52. [Google Scholar] [CrossRef]
- Gentile, L.; Behrens, M.A.; Porcar, L.; Butler, P.; Wagner, N.J.; Olsson, U. Multilamellar vesicle formation from a planar lamellar phase under shear flow. Langmuir 2014, 30, 8316–8325. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Khodaparast, S.; Carroll, J.; Kelly, C.; Robles, E.S.; Cabral, J.T. A microfluidic-multiwell platform for rapid phase mapping of surfactant solutions. Rev. Sci. Instrum. 2020, 91, 045109. [Google Scholar] [CrossRef]
- Trebbin, M.; Steinhauser, D.; Perlich, J.; Buffet, A.; Roth, S.V.; Zimmermann, W.; Thiele, J.; Förster, S. Anisotropic particles align perpendicular to the flow direction in narrow microchannels. Proc. Natl. Acad. Sci. USA 2013, 110, 6706–6711. [Google Scholar] [CrossRef] [Green Version]
- Fürst, C.; Zhang, P.; Roth, S.V.; Drechsler, M.; Förster, S. Self-assembly of block copolymers via micellar intermediate states into vesicles on time scales from milliseconds to days. Polymer 2016, 107, 434–444. [Google Scholar] [CrossRef]
- Lopez, C.G.; Watanabe, T.; Martel, A.; Porcar, L.; Cabral, J.T. Microfluidic-SANS: Flow processing of complex fluids. Sci. Rep. 2015, 5, 7727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamo, M.; Poulos, A.S.; Lopez, C.G.; Martel, A.; Porcar, L.; Cabral, J.T. Droplet microfluidic SANS. Soft Matter 2018, 14, 1759–1770. [Google Scholar] [CrossRef]
- Adamo, M.; Poulos, A.S.; Miller, R.M.; Lopez, C.G.; Martel, A.; Porcar, L.; Cabral, J.T. Rapid contrast matching by microfluidic SANS. Lab Chip 2017, 17, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Pan, R.; Roth, S.; Fӧrster, S. Insights into Growth Kinetics of Colloidal Gold Nanoparticles: In Situ SAXS and UV–Vis Evaluation. J. Phys. Chem. C 2021, 125, 1087–1095. [Google Scholar] [CrossRef]
- Probst, J.; Borca, C.N.; Newton, M.A.; van Bokhoven, J.; Huthwelker, T.; Stavrakis, S.; deMello, A. In Situ X-ray Absorption Spectroscopy and Droplet-Based Microfluidics: An Analysis of Calcium Carbonate Precipitation. ACS Meas. Au 2021, 1, 27–34. [Google Scholar] [CrossRef]
- Brenker, J.; Henzler, K.; Borca, C.N.; Huthwelker, T.; Alan, T. X-ray compatible microfluidics for in situ studies of chemical state, transport and reaction of light elements in an aqueous environment using synchrotron radiation. Lab Chip 2022, 22, 1214–1230. [Google Scholar] [CrossRef]
- Raj, P.M.; Barbe, L.; Andersson, M.; Moreira, M.D.A.; Haase, D.; Wootton, J.; Nehzati, S.; Terry, A.E.; Friel, R.J.; Tenje, M. Fabrication and characterisation of a silicon-borosilicate glass microfluidic device for synchrotron-based hard X-ray spectroscopy studies. RSC Adv. 2021, 11, 29859–29869. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghmur, A.; Hamad, I. Microfluidic Nanomaterial Synthesis and In Situ SAXS, WAXS, or SANS Characterization: Manipulation of Size Characteristics and Online Elucidation of Dynamic Structural Transitions. Molecules 2022, 27, 4602. https://doi.org/10.3390/molecules27144602
Yaghmur A, Hamad I. Microfluidic Nanomaterial Synthesis and In Situ SAXS, WAXS, or SANS Characterization: Manipulation of Size Characteristics and Online Elucidation of Dynamic Structural Transitions. Molecules. 2022; 27(14):4602. https://doi.org/10.3390/molecules27144602
Chicago/Turabian StyleYaghmur, Anan, and Islam Hamad. 2022. "Microfluidic Nanomaterial Synthesis and In Situ SAXS, WAXS, or SANS Characterization: Manipulation of Size Characteristics and Online Elucidation of Dynamic Structural Transitions" Molecules 27, no. 14: 4602. https://doi.org/10.3390/molecules27144602