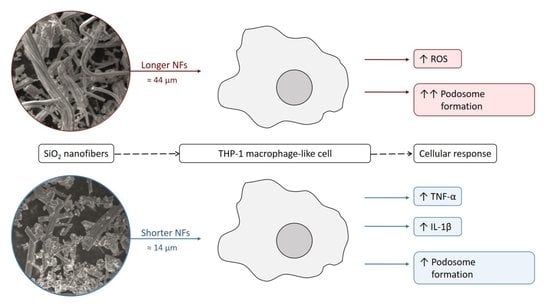
SiO2 Fibers of Two Lengths and Their Effect on Cellular Responses of Macrophage-like Cells
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Monocyte Differentiation
4.2. Nanomaterials
4.3. Cytotoxicity Assays
4.4. ROS Production Assay
4.5. Cytokine Production Assay
4.6. F-Actin Staining and Confocal Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding Cellular Interactions with Nanomaterials: Towards a Rational Design of Medical Nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef] [PubMed]
- Eldawud, R.; Wagner, A.; Dong, C.; Stueckle, T.A.; Rojanasakul, Y.; Dinu, C.Z. Carbon Nanotubes Physicochemical Properties Influence the Overall Cellular Behavior and Fate. NanoImpact 2018, 9, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Kurtz-Chalot, A.; Villiers, C.; Pourchez, J.; Boudard, D.; Martini, M.; Marche, P.N.; Cottier, M.; Forest, V. Impact of Silica Nanoparticle Surface Chemistry on Protein Corona Formation and Consequential Interactions with Biological Cells. Mater. Sci. Eng. C 2017, 75, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Valsesia, A.; Desmet, C.; Ojea-Jiménez, I.; Oddo, A.; Capomaccio, R.; Rossi, F.; Colpo, P. Direct Quantification of Nanoparticle Surface Hydrophobicity. Commun. Chem. 2018, 1, 53. [Google Scholar] [CrossRef]
- Coreas, R.; Cao, X.; DeLoid, G.M.; Demokritou, P.; Zhong, W. Lipid and Protein Corona of Food-Grade TiO2 Nanoparticles in Simulated Gastrointestinal Digestion. NanoImpact 2020, 20, 100272. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Simberg, D. Interactions of Nanoparticles with Plasma Proteins: Implication on Clearance and Toxicity of Drug Delivery Systems. Expert Opin. Drug Deliv. 2011, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and Identification of Soft Corona Proteins at Nanoparticles and Their Impact on Cellular Association. Nat. Commun. 2020, 11, 4535. [Google Scholar] [CrossRef]
- Kupcik, R.; Macak, J.M.; Rehulkova, H.; Sopha, H.; Fabrik, I.; Anitha, V.C.; Klimentova, J.; Murasova, P.; Bilkova, Z.; Rehulka, P. Amorphous TiO2 Nanotubes as a Platform for Highly Selective Phosphopeptide Enrichment. ACS Omega 2019, 4, 12156–12166. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Li, X.; Zhu, M.; Yu, H.; Chu, R.; Chen, W.; Wang, B.; Wang, M.; Zheng, L.; Chai, Z.; et al. Hepatic Impacts of Gold Nanoparticles with Different Surface Coatings as Revealed by Assessing the Hepatic Drug-Metabolizing Enzyme and Lipid Homeostasis in Mice. NanoImpact 2020, 20, 100259. [Google Scholar] [CrossRef]
- Zheng, H.; Mortensen, L.J.; Ravichandran, S.; Bentley, K.; DeLouise, L.A. Effect of Nanoparticle Surface Coating on Cell Toxicity and Mitochondria Uptake. J. Biomed. Nanotechnol. 2017, 13, 155–166. [Google Scholar] [CrossRef]
- Sakai, N.; Matsui, Y.; Nakayama, A.; Tsuda, A.; Yoneda, M. Functional-Dependent and Size-Dependent Uptake of Nanoparticles in PC12. J. Phys. Conf. Ser. 2011, 304, 012049. [Google Scholar] [CrossRef]
- Ichikawa, S.; Shimokawa, N.; Takagi, M.; Kitayama, Y.; Takeuchi, T. Size-Dependent Uptake of Electrically Neutral Amphipathic Polymeric Nanoparticles by Cell-Sized Liposomes and an Insight into Their Internalization Mechanism in Living Cells. Chem. Commun. 2018, 54, 4557–4560. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, J.; Fan, X.; Gao, H. Size and Shape Effects on Diffusion and Absorption of Colloidal Particles near a Partially Absorbing Sphere: Implications for Uptake of Nanoparticles in Animal Cells. Phys. Rev. E 2008, 78, 061914. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of Nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.F.; Wu, N.; Porter, D.; Buford, M.; Wolfarth, M.; Holian, A. Particle Length-Dependent Titanium Dioxide Nanomaterials Toxicity and Bioactivity. Part. Fibre Toxicol. 2009, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Schinwald, A.; Murphy, F.A.; Prina-Mello, A.; Poland, C.A.; Byrne, F.; Movia, D.; Glass, J.R.; Dickerson, J.C.; Schultz, D.A.; Jeffree, C.E.; et al. The Threshold Length for Fiber-Induced Acute Pleural Inflammation: Shedding Light on the Early Events in Asbestos-Induced Mesothelioma. Toxicol. Sci. 2012, 128, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Cacchioli, A.; Ravanetti, F.; Alinovi, R.; Pinelli, S.; Rossi, F.; Negri, M.; Bedogni, E.; Campanini, M.; Galetti, M.; Goldoni, M.; et al. Cytocompatibility and Cellular Internalization Mechanisms of SiC/SiO2 Nanowires. Nano Lett. 2014, 14, 4368–4375. [Google Scholar] [CrossRef]
- Ahmad, H. Biocompatible SiO2 in the Fabrication of Stimuli-Responsive Hybrid Composites and Their Application Potential. J. Chem. 2015, 2015, e846328. [Google Scholar] [CrossRef]
- Lovětinská-Šlamborová, I.; Holý, P.; Exnar, P.; Veverková, I. Silica Nanofibers with Immobilized Tetracycline for Wound Dressing. J. Nanomater. 2016, 2016, 2485173. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhao, C.; Shan, H.; Jiao, Y.; Zhang, Q.; Li, X.; Yu, J.; Ding, B. Deoxycholic Acid-Modified Microporous SiO2 Nanofibers Mimicking Colorectal Microenvironment to Optimize Radiotherapy-Chemotherapy Combined Therapy. Biomed. Mater. 2021, 16, 065020. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Sadeghi-Soureh, S.; Amirsaadat, S.; Pourpirali, R.; Alijani, S. Dual Drug Release Mechanisms through Mesoporous Silica Nanoparticle/Electrospun Nanofiber for Enhanced Anticancer Efficiency of Curcumin. J. Biomed. Mater. Res. Part A 2022, 110, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V. Silica Nanoparticles in Transmucosal Drug Delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, T.; Jia, L.; Chen, S.; Huang, D.; Chen, W. Synthesis and Drug Delivery Property of Silica Nanotubes Prepared Using Gelatin Nanofibers as Novel Sacrificed Template. Mater. Lett. 2017, 209, 334–337. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent Advances in the Application of Mesoporous Silica-Based Nanomaterials for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110267. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Forman, H.J.; Torres, M. Redox Signaling in Macrophages. Mol. Asp. Med. 2001, 22, 189–216. [Google Scholar] [CrossRef]
- Widdrington, J.D.; Gomez-Duran, A.; Pyle, A.; Ruchaud-Sparagano, M.-H.; Scott, J.; Baudouin, S.V.; Rostron, A.J.; Lovat, P.E.; Chinnery, P.F.; Simpson, A.J. Exposure of Monocytic Cells to Lipopolysaccharide Induces Coordinated Endotoxin Tolerance, Mitochondrial Biogenesis, Mitophagy, and Antioxidant Defenses. Front. Immunol. 2018, 9, 2217. [Google Scholar] [CrossRef]
- Ott, L.W.; Resing, K.A.; Sizemore, A.W.; Heyen, J.W.; Cocklin, R.R.; Pedrick, N.M.; Woods, H.C.; Chen, J.Y.; Goebl, M.G.; Witzmann, F.A.; et al. Tumor Necrosis Factor-α- and Interleukin-1-Induced Cellular Responses: Coupling Proteomic and Genomic Information. J. Proteome Res. 2007, 6, 2176–2185. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.G.; Matsudaira, P. Structure and Dynamics of Macrophage Podosomes. Eur. J. Cell Biol. 2006, 85, 145–149. [Google Scholar] [CrossRef]
- Hromádko, L.; Koudelková, E.; Bulánek, R.; Macák, J.M. SiO2 Fibers by Centrifugal Spinning with Excellent Textural Properties and Water Adsorption Performance. ACS Omega 2017, 2, 5052–5059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental Study of Nanofiber Production through Forcespinning. J. Appl. Phys. 2013, 113, 024318. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ao, C.; Niu, Y.; Zhang, X.; He, X.; Zhang, W.; Lu, C. Fabrication and Characterization of Electrospun Cellulose/Nano-Hydroxyapatite Nanofibers for Bone Tissue Engineering. Int. J. Biol. Macromol. 2017, 97, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sakai, S.; Kawakami, K. Application of Silicate Electrospun Nanofibers for Cell Culture. J. Sol-Gel Sci. Technol. 2008, 48, 350–355. [Google Scholar] [CrossRef]
- Cavo, M.; Serio, F.; Kale, N.; D’Amone, E.; Gigli, G.; del Mercato, L.L. Electrospun Nanofibers in Cancer Research: From Engineering of in Vitro 3D Cancer Models to Therapy. Biomater. Sci. 2020, 8, 4887–4905. [Google Scholar] [CrossRef]
- Hardick, O.; Dods, S.; Stevens, B.; Bracewell, D.G. Nanofiber Adsorbents for High Productivity Continuous Downstream Processing. J. Biotechnol. 2015, 213, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.-M.; Lee, S.-M.; Michler, G.H.; Roggendorf, H.; Gösele, U.; Knez, M. Nanostructured Pure Anatase Titania Tubes Replicated from Electrospun Polymer Fiber Templates by Atomic Layer Deposition. Chem. Mater. 2008, 20, 3085–3091. [Google Scholar] [CrossRef]
- Rihova, M.; Yurkevich, O.; Motola, M.; Hromadko, L.; Spotz, Z.; Zazpe, R.; Knez, M.; Macak, J.M. ALD Coating of Centrifugally Spun Polymeric Fibers and Postannealing: Case Study for Nanotubular TiO2 Photocatalyst. Nanoscale Adv. 2021, 3, 4589–4596. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The Choice of Phorbol 12-Myristate 13-Acetate Differentiation Protocol Influences the Response of THP-1 Macrophages to a pro-Inflammatory Stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the State of Differentiation of THP-1 Cells Induced by Phorbol Ester and 1,25-Dihydroxyvitamin D3. J. Leukoc. Biol. 1996, 59, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.M.; Kim, H.; Subbannayya, Y.; Giambelluca, M.; Bösl, K.; Kandasamy, R.K. Dose-Dependent Phorbol 12-Myristate-13-Acetate-Mediated Monocyte-to-Macrophage Differentiation Induces Unique Proteomic Signatures in THP-1 Cells. bioRxiv 2020. [CrossRef] [Green Version]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In Vitro Biological Responses to Nanofibrillated Cellulose by Human Dermal, Lung and Immune Cells: Surface Chemistry Aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Boonrungsiman, S.; Suchaoin, W.; Chetprayoon, P.; Viriya-empikul, N.; Aueviriyavit, S.; Maniratanachote, R. Shape and Surface Properties of Titanate Nanomaterials Influence Differential Cellular Uptake Behavior and Biological Responses in THP-1 Cells. Biochem. Biophys. Rep. 2017, 9, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D. The Effect of Size of Materials Formed or Implanted In Vivo on the Macrophage Response and the Resultant Influence on Clinical Outcome. Materials 2021, 14, 4572. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Jones, W.; Rojanasakul, Y.; Cheng, N.; Schwegler-Berry, D.; Baron, P.; Deye, G.J.; Li, C.; Castranova, V. Critical Role of Glass Fiber Length in TNF-Alpha Production and Transcription Factor Activation in Macrophages. Am. J. Physiol. 1999, 276, L426–L434. [Google Scholar] [CrossRef]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative Analysis of the Role of Fiber Length on Phagocytosis and Inflammatory Response by Alveolar Macrophages. Biochim. Biophys. Acta 2017, 1861, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Miller, Y.I.; Worrall, D.S.; Funk, C.D.; Feramisco, J.R.; Witztum, J.L. Actin Polymerization in Macrophages in Response to Oxidized LDL and Apoptotic Cells: Role of 12/15-Lipoxygenase and Phosphoinositide 3-Kinase. Mol. Biol. Cell 2003, 14, 4196–4206. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smela, D.; Chang, C.-J.; Hromadko, L.; Macak, J.; Bilkova, Z.; Taniguchi, A. SiO2 Fibers of Two Lengths and Their Effect on Cellular Responses of Macrophage-like Cells. Molecules 2022, 27, 4456. https://doi.org/10.3390/molecules27144456
Smela D, Chang C-J, Hromadko L, Macak J, Bilkova Z, Taniguchi A. SiO2 Fibers of Two Lengths and Their Effect on Cellular Responses of Macrophage-like Cells. Molecules. 2022; 27(14):4456. https://doi.org/10.3390/molecules27144456
Chicago/Turabian StyleSmela, Denisa, Chia-Jung Chang, Ludek Hromadko, Jan Macak, Zuzana Bilkova, and Akiyoshi Taniguchi. 2022. "SiO2 Fibers of Two Lengths and Their Effect on Cellular Responses of Macrophage-like Cells" Molecules 27, no. 14: 4456. https://doi.org/10.3390/molecules27144456