New Alk(en)ylhydroxycyclohexanes with Tyrosinase Inhibition Potential from Harpephyllum caffrum Bernh. Gum Exudate
Abstract
:1. Introduction
2. Results and Discussion
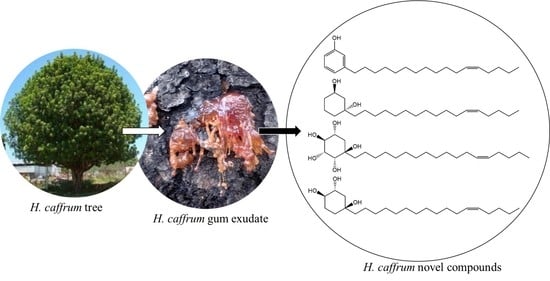
2.1. Chemistry of Isolated Compounds
2.2. Tyrosinase Inhibitory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Sample Collection and Identification
3.3. Extraction and Purification
3.4. Tyrosinase Inhibitory Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Umadevi, I.; Daniel, M.; Sabnis, S. Chemotaxonomic studies on some members of Anacardiaceae. Proc. Plant Sci. 1988, 98, 205–208. [Google Scholar] [CrossRef]
- Pell, S.K. Molecular Systematics of The Cashew Family (Anacardiaceae). Ph.D Thesis, Louisiana State University, Baton Rouge, LA, USA, 2004. [Google Scholar]
- Hutchings, A. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996; p. 464. [Google Scholar]
- Van Wyk, B.E.; van Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa; Briza Publication: Pretoria, South Africa, 2002; p. 304. [Google Scholar]
- El Sherbeiny, A.; El Ansari, M. The polyphenolics and flavonoids of Harpephyllum caffrum. Planta Med. 1976, 29, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Shabana, M.M.; El Sayed, A.M.; Yousif, M.F.; El Sayed, A.M.; Sleem, A.A. Bioactive constituents from Harpephyllum caffrum Bernh. and Rhus coriaria L. Pharmacogn. Mag. 2011, 7, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodley, R.; Koorbanally, N.A.; Shahidul Islam, M.; Jonnalagadda, S.B. Structure and antioxidant activity of phenolic compounds isolated from the edible fruits and stem bark of Harpephyllum caffrum. J. Environ. Sci. Health B 2014, 49, 938–944. [Google Scholar] [CrossRef]
- Choudhary, P.D.; Pawar, H.A. Recently investigated natural gums and mucilages as pharmaceutical excipients: An overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.; Tyagi, S.; Gupta, R.K.; Tyagi, Y.K. Natural gums of plant origin as edible coatings for food industry applications. Crit. Rev. Biotechnol. 2017, 37, 959–973. [Google Scholar] [CrossRef]
- Jani, G.K.; Shah, D.P.; Prajapati, V.D.; Jain, V.C. Gums and mucilages: Versatile excipients for pharmaceutical formulations. Asian J. Pharm. Sci. 2009, 4, 309–323. [Google Scholar]
- Rigano, L.; Deola, M.; Zaccariotto, F.; Colleoni, T.; Lionetti, N. A new gelling agent and rheology modifier in cosmetics: Caesalpinia spinosa gum. Cosmetics 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Mirhosseini, H.; Amid, B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef]
- Bekana, D.; Kebede, T.; Assefa, M.; Kassa, H. Comparative phytochemical analyses of resins of Boswellia species (B. papyrifera (Del.) Hochst., B. neglecta S. Moore, and B. rivae Engl.) from northwestern, southern, and southeastern Ethiopia. Int. Sch. Res. Not. 2014, 2014, 374678. [Google Scholar] [CrossRef] [Green Version]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- Okoth, D.A.; Akala, H.M.; Johnson, J.D.; Koorbanally, N.A. Alkyl phenols, alkenyl cyclohexenones and other phytochemical constituents from Lannea rivae (chiov) Sacleux (Anacardiaceae) and their bioactivity. Med. Chem. Res. 2016, 25, 690–703. [Google Scholar] [CrossRef]
- Roumy, V.; Fabre, N.; Portet, B.; Bourdy, G.; Acebey, L.; Vigor, C.; Valentin, A.; Moulis, C. Four anti-protozoal and anti-bacterial compounds from Tapirira guianensis. Phytochemistry 2009, 70, 305–311. [Google Scholar] [CrossRef]
- Laurent, P.; Hamdani, A.; Braekman, J.C.; Daloze, D.; Isbell, L.A.; De Biseau, J.C.; Pasteels, J.M. New 1-alk (en) yl-1, 3, 5-trihydroxycyclohexanes from the Dufour gland of the African ant Crematogaster nigriceps. Tetrahedron Lett. 2003, 44, 1383–1386. [Google Scholar] [CrossRef]
- Yuliana, M.; Nguyen-Thi, B.T.; Faika, S.; Huynh, L.H.; Soetaredjo, F.E.; Ju, Y.H. Separation and purification of cardol, cardanol and anacardic acid from cashew (Anacardium occidentale L.) nut-shell liquid using a simple two-step column chromatography. J. Taiwan Inst. Chem. Eng. 2014, 45, 2187–2193. [Google Scholar] [CrossRef] [Green Version]
- Knödler, M.; Conrad, J.; Wenzig, E.M.; Bauer, R.; Lacorn, M.; Beifuss, U.; Carle, R.; Schieber, A. Anti-inflammatory 5-(11′ Z-heptadecenyl)-and 5-(8′ Z, 11′ Z-heptadecadienyl)-resorcinols from mango (Mangifera indica L.) peels. Phytochemistry 2008, 69, 988–993. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family–A review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Yu, X.P.; Su, W.C.; Wang, Q.; Zhuang, J.X.; Tong, R.Q.; Chen, Q.X.; Chen, Q.H. Inhibitory mechanism of cardanols on tyrosinase. Process Biochem. 2016, 51, 2230–2237. [Google Scholar] [CrossRef]
- Mapunya, M.B.; Nikolova, R.V.; Lall, N. Melanogenesis and antityrosinase activity of selected South African plants. Evid. Based Complement. Altern. Med. 2012, 2012, 374017. [Google Scholar] [CrossRef]
- Lall, N.; Blom van Staden, A.; Rademan, S.; Lambrechts, I.; De Canha, M.N.; Mahore, J.; Winterboer, S.; Twilley, D. Antityrosinase and anti-acne potential of plants traditionally used in the Jongilanga community in Mpumalanga. S. Afr. J. Bot. 2019, 126, 241–249. [Google Scholar] [CrossRef]




| Position | 1 | 2 | 3 | 4 | * 5 | ** 6 | ||
|---|---|---|---|---|---|---|---|---|
| CDCl3 | CD3OD | CDCl3 | ||||||
| 1 | 1 | 4.07, bs | 3.91, m | 3.51, bs | 3.46, bs | 3.41, dd (10.5, 4.6) | ||
| 2 | 2 | 6.63, s | 1.83, m & 1.48, m | 4.01, bs | 3.90, m | 3.85, m | 1.91, bd (14.4) & 1.45, m | 4.01, bs |
| 3 | 3 | 4.47, bs | 1.46–1.57, m | 1.45–1.50, m | 4.31, bs | 1.96–1.98, m & 1.45, m | ||
| 4 | 4 | 6.74, d (7.5) | 1.38, bd (1.86) | 3.41, bd (9.8) | 2.29, bd (12.5) & 1.40, m | |||
| 5 | 5 | 7.11, t (7.9) | 1.95, m & 1.43, m | 1.89–1.97, m & 1.66, m | 1.89–1.93, dt (13.9, 3.0) & 1.62–1.64, m | 4.35, tt (11.4, 4.2) | 1.86, m & 1.62, m | |
| 6 | 6 | 6.61, dd (8.4, 2.3) | 1.60, m & 1.35, m | 1.93, m & 1.22, m | 1.74, bd (12.0) | 1.78, dd (12.9, 4.0) | 2.11, bd (12.5) & 1.32, m | 1.53, m |
| 1′ | 1′ | 2.54, t (7.5) | 1.75, m & 1.44, m | 1.42, m | 1.66–1.67, m & 1.54–1.59, m | 1.48, m & 1.38, m | 1.84, m & 1.48, m | |
| 2′–10′ | 2′–14′ | 1.24–1.57, m | 1.23–1.32, m | 1.10–1.26, m & 1.93 | 1.29–1.36, m | 1.30–1.50, m | 1.20–1.30, m | |
| 11′ | 15′ | 1.99, m | 1.96–1.99, m | 1.23–1.44, m | 1.96–2.01, m | 2.02–2.05, m | 2.03, m | 1.98–2.02, m |
| 12′ | 16′ | 5.34, m | 5.31, m | 1.96–1.99, m | 5.32, m | 5.36, m | 5.37, m | 5.32, t (5.0) |
| 13′ | 17′ | 5.34, m | 5.31, m | 5.32, m | 5.32, m | 5.36, m | 5.37, m | 5.32, t (5.0) |
| 14′ | 18′ | 1.99, m | 1.96–1.99, m | 5.32, m | 1.96–2.01, m | 2.02–2.05, m | 2.03, m | 1.98–2.02, m |
| 15′ | 19′ | 1.24–1.30, m | 1.28–1.31, m | 1.96–1.99, m | 1.29–1.36, m | 1.30–1.50, m | 1.20–1.30, m | |
| 16′ | 20′ | 1.24–1.30, m | 1.28–1.31, m | 1.31, m | 1.30 1.42–1.44, m | 1.30–1.50, m | 1.20–1.30, m | |
| 17′ | 21′ | 0.88, t (6.8) | 0.87, t (6.8) | 1.28–1.31, m | 0.89, t (5.7) | 0.92, t (6.9) | 0.91 (6.6) | 0.86, t (6.8) |
| 18′ | 0.86, t (6.8) | |||||||
| Position | 1 | 2 | 4 | Position | 3 |
|---|---|---|---|---|---|
| 1 | 155.4, C | 67.8, CH | 70.9, CH | 1 | 67.6, CH |
| 2 | 115.3, CH | 41.2, CH2 | 67.7, CH | 2 | 66.8, CH |
| 3 | 144.9, C | 72.9, C | 40.6, CH2 | 3 | 65.9, CH |
| 4 | 120.9, CH | 43.7, CH2 | 76.1, C | 4 | 72.7, CH |
| 5 | 129.3, CH | 16.0 | 28.3, CH2 | 5 | 73.6, C |
| 6 | 112.4, CH | 36.9 | 41.9, CH2 | 6 | 45.9, CH2 |
| 1′ | 35.8, CH2 | 32.8, CH2 | 29.8, CH2 | 1′ | 44.5, CH2 |
| 2′ | 31.3, CH2 | 22.8, CH2 | 23.3, CH2 | 2′ | 35.3, CH2 |
| 11′ | 27.2, CH2 | 27.2, CH2 | 28.1, CH2 | 12′ | 27.2, CH2 |
| 12′ | 129.8, CH | 129.8, CH | 130.8, CH | 13′ | 129.8, CH |
| 13′ | 129.9, CH | 129.9, CH | 130.8, CH | 14′ | 129.9, CH |
| 14′ | 26.9, CH2 | 26.9, CH2 | 27.9, CH2 | 15′ | 26.9, CH2 |
| 15′ | 31.9, CH2 | 31.9, CH2 | 33.1, CH2 | 16′ | 31.9, CH2 |
| 16′ | 22.3, CH2 | 22.3, CH2 | 23.1, CH2 | 17′ | 22.3, CH2 |
| 17′ | 14.0, CH3 | 13.9, CH3 | 14.3, CH3 | 18′ | 13.9, CH3 |
| Test Samples | Anti-Tyrosinase IC50 ± SD (µg/mL) | Correlation Coefficient (R2) |
|---|---|---|
| HCG-EtOH | b 11.32 ± 0.80 | 0.9892 |
| Compound 1 | e 41.77 ± 0.62 | 0.9813 |
| Compound 2 | c 24.90 ± 1.10 | 0.9470 |
| Compound 3 | c 26.99 ± 1.30 | 0.9659 |
| Compound 4 | d 34.90 ± 0.73 | 0.9731 |
| Arbutin | b 9.85 ± 0.42 | 0.9577 |
| Kojic acid | a 4.34 ± 0.37 | 0.9969 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodede, O.; More, G.K.; Moodley, R.; Steenkamp, P.; Baijnath, H.; Maharaj, V.; Prinsloo, G. New Alk(en)ylhydroxycyclohexanes with Tyrosinase Inhibition Potential from Harpephyllum caffrum Bernh. Gum Exudate. Molecules 2022, 27, 3839. https://doi.org/10.3390/molecules27123839
Bodede O, More GK, Moodley R, Steenkamp P, Baijnath H, Maharaj V, Prinsloo G. New Alk(en)ylhydroxycyclohexanes with Tyrosinase Inhibition Potential from Harpephyllum caffrum Bernh. Gum Exudate. Molecules. 2022; 27(12):3839. https://doi.org/10.3390/molecules27123839
Chicago/Turabian StyleBodede, Olusola, Garland K. More, Roshila Moodley, Paul Steenkamp, Himansu Baijnath, Vinesh Maharaj, and Gerhard Prinsloo. 2022. "New Alk(en)ylhydroxycyclohexanes with Tyrosinase Inhibition Potential from Harpephyllum caffrum Bernh. Gum Exudate" Molecules 27, no. 12: 3839. https://doi.org/10.3390/molecules27123839