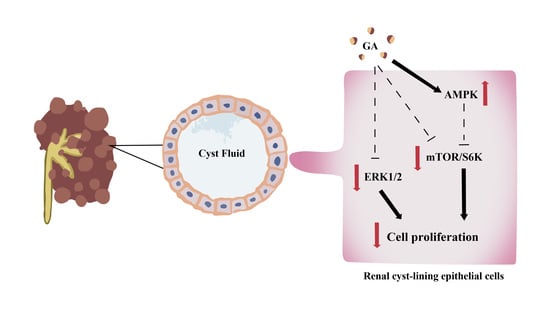
Potential Application of Gambogic Acid for Retarding Renal Cyst Progression in Polycystic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Compounds
2.2. Cell Cultures and Treatments
2.3. MDCK Cyst Experiments
2.4. Cell Viability Assay
2.5. Cell Proliferation Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Gambogic Acid Had No Cytotoxic Effect on MDCK Cell Viability
3.2. Gambogic Acid Slowed MDCK Cyst Progression
3.3. Gambogic Acid Suppressed MDCK and Pkd1 Mutant Cell Proliferation
3.4. Gambogic Acid Inhibited Phosphorylation of ERK1/2 and mTOR/S6K Protein Expression in MDCK and Pkd1 Mutant Cells
3.5. Gambogic Acid Upregulated the AMPK Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chebib, F.T.; Perrone, R.D.; Chapman, A.B.; Dahl, N.K.; Harris, P.C.; Mrug, M.; Mustafa, R.A.; Rastogi, A.; Watnick, T.; Yu, A.S.L.; et al. A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. J. Am. Soc. Nephrol. JASN 2018, 29, 2458–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhia, R.; Mishra, A.; Biggers, L.; Malladi, V.; Cobo-Stark, P.; Hajarnis, S.; Patel, V. Enhancer and super-enhancer landscape in polycystic kidney disease. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hajarnis, S.; Lakhia, R.; Yheskel, M.; Williams, D.; Sorourian, M.; Liu, X.; Aboudehen, K.; Zhang, S.; Kersjes, K.; Galasso, R.; et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 2017, 8, 14395. [Google Scholar] [CrossRef]
- Chapin, H.C.; Caplan, M.J. The cell biology of polycystic kidney disease. J. Cell Biol. 2010, 191, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Wallace, D.P. Cyclic AMP-mediated cyst expansion. Biochim. Biophys. Acta 2011, 1812, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- Shibazaki, S.; Yu, Z.; Nishio, S.; Tian, X.; Thomson, R.B.; Mitobe, M.; Louvi, A.; Velazquez, H.; Ishibe, S.; Cantley, L.G.; et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 2008, 17, 1505–1516. [Google Scholar] [CrossRef] [Green Version]
- Shillingford, J.M.; Murcia, N.S.; Larson, C.H.; Low, S.H.; Hedgepeth, R.; Brown, N.; Flask, C.A.; Novick, A.C.; Goldfarb, D.A.; Kramer-Zucker, A.; et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5466–5471. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Xu, Y.; Fan, S.; Meng, J.; Shen, X.; Xiao, Q.; Li, Y.; Zhang, L.; Zhang, X.; Wu, G.; et al. Canonical Wnt inhibitors ameliorate cystogenesis in a mouse ortholog of human ADPKD. JCI Insight 2018, 3, e95874. [Google Scholar] [CrossRef] [Green Version]
- Yuajit, C.; Chatsudthipong, V. Nutraceutical for Autosomal Dominant Polycystic Kidney Disease Therapy. J. Med. Assoc. Thail. 2016, 99 (Suppl. S1), S97–S103. [Google Scholar]
- Liesenklas, W.; Auterhoff, H. The constitution of gambogic acid and its isomerization. 4. Chemistry of gum-resin. Arch. Pharm. Ber. Dtsch. Pharm. Ges. 1966, 299, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Du, J.; Kong, D.; Yang, G.; Zhou, Q.; You, F.; Lin, Y.; Wang, Y. Gambogic acid inhibits proliferation and induces apoptosis of human acute T-cell leukemia cells by inducing autophagy and downregulating β-catenin signaling pathway: Mechanisms underlying the effect of Gambogic acid on T-ALL cells. Oncol. Rep. 2020, 44, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Lin, L.; Li, H. Gambogic Acid as a Candidate for Cancer Therapy: A Review. Int. J. Nanomed. 2020, 15, 10385–10399. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, Q.; Wang, X.M.; Li, G.X.; Shen, S.; Wei, X.L. Gambogic acid exhibits anti-metastatic activity on malignant melanoma mainly through inhibition of PI3K/Akt and ERK signaling pathways. Eur. J. Pharmacol. 2019, 864, 172719. [Google Scholar] [CrossRef]
- Yi, T.; Yi, Z.; Cho, S.-G.; Luo, J.; Pandey, M.K.; Aggarwal, B.B.; Liu, M. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008, 68, 1843–1850. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.L.; Yang, D.Z.; Lv, C. Anti-inflammatory effects of gambogic acid in murine collagen--induced arthritis through PI3K/Akt signaling pathway. Mol. Med. Rep. 2018, 17, 4791–4796. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Wang, H.J.; Zhao, W.W.; Sun, Y.L.; Hu, L.K. Gambogic acid improves non-small cell lung cancer progression by inhibition of mTOR signaling pathway. Kaohsiung J. Med. Sci. 2017, 33, 543–549. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.; Yao, W.; Yang, Z.; Gao, P.; Liu, M.; Wang, H.; Chen, S.; Wang, D.; Wang, W.; et al. Gambogic acid affects ESCC progression through regulation of PI3K/AKT/mTOR signal pathway. J. Cancer 2020, 11, 5568–5577. [Google Scholar] [CrossRef]
- He, X.Y.; Liu, X.J.; Chen, X.; Bian, L.G.; Zhao, W.G.; Shen, J.K.; Sun, Q.F. Gambogic acid induces EGFR degradation and Akt/mTORC1 inhibition through AMPK dependent-LRIG1 upregulation in cultured U87 glioma cells. Biochem. Biophys. Res. Commun. 2013, 435, 397–402. [Google Scholar] [CrossRef]
- Yuajit, C.; Muanprasat, C.; Homvisasevongsa, S.; Chatsudthipong, V. Steviol stabilizes polycystin 1 expression and promotes lysosomal degradation of CFTR and beta-catenin proteins in renal epithelial cells. Biomed. Pharmacother. 2017, 94, 820–826. [Google Scholar] [CrossRef]
- Margaria, J.P.; Campa, C.C.; De Santis, M.C.; Hirsch, E.; Franco, I. The PI3K/Akt/mTOR pathway in polycystic kidney disease: A complex interaction with polycystins and primary cilium. Cell. Signal. 2020, 66, 109468. [Google Scholar] [CrossRef] [PubMed]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathomthongtaweechai, N.; Soodvilai, S.; Pichyangkura, R.; Muanprasat, C. Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules 2020, 25, 5589. [Google Scholar] [CrossRef] [PubMed]
- Veeraphan, P.; Chavasiri, W.; Muanprasat, C.; Chatsudthipong, V.; Yuajit, C. A chalcone derivative retards renal cyst enlargement by inhibiting fluid secretion and cell proliferation in an in vitro model of polycystic kidney disease. Clin. Exp. Nephrol. 2021, 25, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Yuajit, C.; Muanprasat, C.; Gallagher, A.R.; Fedeles, S.V.; Kittayaruksakul, S.; Homvisasevongsa, S.; Somlo, S.; Chatsudthipong, V. Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem. Pharmacol. 2014, 88, 412–421. [Google Scholar] [CrossRef]
- Leonhard, W.N.; van der Wal, A.; Novalic, Z.; Kunnen, S.J.; Gansevoort, R.T.; Breuning, M.H.; de Heer, E.; Peters, D.J. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkd1-deletion model. Am. J. Physiology. Ren. Physiol. 2011, 300, F1193–F1202. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Gao, J.; Zhou, L.; Li, X.; Li, W.; Li, X.; Xia, Y.; Yang, B. Ginkgolide B inhibits renal cyst development in in vitro and in vivo cyst models. Am. J. Physiol. Ren. Physiol. 2012, 302, F1234–F1242. [Google Scholar] [CrossRef] [Green Version]
- Yuajit, C.; Homvisasevongsa, S.; Chatsudthipong, L.; Soodvilai, S.; Muanprasat, C.; Chatsudthipong, V. Steviol reduces MDCK Cyst formation and growth by inhibiting CFTR channel activity and promoting proteasome-mediated CFTR degradation. PLoS ONE 2013, 8, e58871. [Google Scholar] [CrossRef] [Green Version]
- Kale, V.P.; Gilhooley, P.J.; Phadtare, S.; Nabavizadeh, A.; Pandey, M.K. Role of Gambogic Acid in Chemosensitization of Cancer. In Role of Nutraceuticals in Cancer Chemosensitization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–167. [Google Scholar]
- Qi, Q.; Lu, N.; Wang, X.-T.; Gu, H.-Y.; Yang, Y.; Liu, W.; Li, C.; You, Q.-D.; Guo, Q.-L. Anti-invasive effect of gambogic acid in MDA-MB-231 human breast carcinoma cells. Biochem. Cell Biol. 2008, 86, 386–395. [Google Scholar] [CrossRef]
- Song, X.; Tsakiridis, E.; Steinberg, G.R.; Pei, Y. Targeting AMP-activated protein kinase (AMPK) for treatment of autosomal dominant polycystic kidney disease. Cell. Signal. 2020, 73, 109704. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Su, J.; Xu, G.; Zhao, L.; Qi, Q. Involvement of E-cadherin/AMPK/mTOR axis in LKB1-induced sensitivity of non-small cell lung cancer to gambogic acid. Biochem. Pharmacol. 2019, 169, 113635. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khunpatee, N.; Bhukhai, K.; Chatsudthipong, V.; Yuajit, C. Potential Application of Gambogic Acid for Retarding Renal Cyst Progression in Polycystic Kidney Disease. Molecules 2022, 27, 3837. https://doi.org/10.3390/molecules27123837
Khunpatee N, Bhukhai K, Chatsudthipong V, Yuajit C. Potential Application of Gambogic Acid for Retarding Renal Cyst Progression in Polycystic Kidney Disease. Molecules. 2022; 27(12):3837. https://doi.org/10.3390/molecules27123837
Chicago/Turabian StyleKhunpatee, Nutchanard, Kanit Bhukhai, Varanuj Chatsudthipong, and Chaowalit Yuajit. 2022. "Potential Application of Gambogic Acid for Retarding Renal Cyst Progression in Polycystic Kidney Disease" Molecules 27, no. 12: 3837. https://doi.org/10.3390/molecules27123837