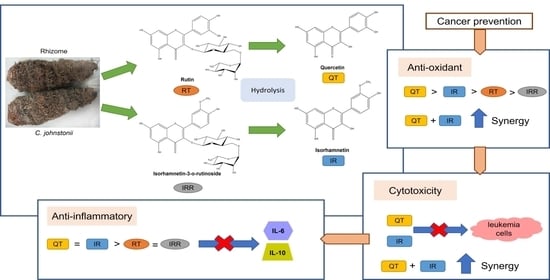
Hydrolyzed Flavonoids from Cyrtosperma johnstonii with Superior Antioxidant, Antiproliferative, and Anti-Inflammatory Potential for Cancer Prevention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Standardization of Sample Plant Extracts by HPLC Analysis
2.3. Determination of the Antioxidant Activity of Individual Compounds
2.4. Determination of the Antioxidant Activity of Combined Compounds
2.5. Cytotoxicity Study against Cancer Cells
2.6. Cytotoxicity against Normal Cells
2.7. Determination of Pro- and Anti-Inflammatory Activity by the Enzyme-Linked Immunosorbent (ELISA) Assay
2.8. Statistical Analysis
3. Results
3.1. Standardization of Sample Plant Extracts by HPLC Analysis
3.2. Determination of Antioxidant Activities of Individual Compounds
3.3. Determination of the Antioxidant Activity of Combined Compounds
3.4. Test of Cytotoxicity against Normal Cells
3.5. Cytotoxicity Study against Cancer Cells
3.6. Cytotoxicity Study against Cancer Cells in Combination
3.7. Determination of Pro- and Anti-Inflammatory Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S.; Leklob, A.; Pongnikorn, D.; Rozek, L.S.; Siriarechakul, S.; et al. National and Subnational Population-Based Incidence of Cancer in Thailand: Assessing Cancers with the Highest Burdens. Cancers 2017, 9, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1. [Google Scholar]
- Li, K.; Lv, X.X.; Hua, F.; Lin, H.; Sun, W.; Cao, W.B.; Fu, X.M.; Xie, J.; Yu, J.J.; Li, Z.; et al. Targeting acute myeloid leukemia with a proapoptotic peptide conjugated to a Toll-like receptor 2-mediated cell-penetrating peptide. Int. J. Cancer 2014, 134, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Gessler, M.; Poustka, A.; Cavenee, W.; Neve, R.L.; Orkin, S.H.; Bruns, G.A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature 1990, 343, 774–778. [Google Scholar] [CrossRef]
- Oberley, T.D. Oxidative damage and cancer. Am. J. Pathol. 2002, 160, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88-and TRIF-dependent signaling pathways of Toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochemical pharmacology 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Okonogi, S.; Khonkarn, R.; Mankhetkorn, S.; Unger, F.M.; Viernstein, H. Antioxidant activity and cytotoxicity of Cyrtosperma johnstonii extracts on drug sensitive and resistant leukemia and small cell lung carcinoma cells. Pharm. Biol. 2013, 51, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Menezes, J.C.; Orlikova, B.; Morceau, F.; Diederich, M. Natural and Synthetic Flavonoids: Structure-Activity Relationship and Chemotherapeutic Potential for the Treatment of Leukemia. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S4–S28. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov. Ther. 2015, 9, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Anuchapreeda, S.; Chueahongthong, F.; Viriyaadhammaa, N.; Panyajai, P.; Anzawa, R.; Tima, S.; Ampasavate, C.; Saiai, A.; Rungrojsakul, M.; Usuki, T. Antileukemic cell proliferation of active compounds from kaffir lime (Citrus hystrix) leaves. Molecules 2020, 25, 1300. [Google Scholar] [CrossRef] [Green Version]
- Tima, S.; Anuchapreeda, S.; Ampasavate, C.; Berkland, C.; Okonogi, S. Stable curcumin-loaded polymeric micellar formulation for enhancing cellular uptake and cytotoxicity to FLT3 overexpressing EoL-1 leukemic cells. Eur. J. Pharm. Biopharm. 2017, 114, 57–68. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Mueller, M.; Janngeon, K.; Puttipan, R.; Unger, F.M.; Viernstein, H.; Okonogi, S. Anti-inflammatory, antibacterial, and antioxidant activities of Thai medicinal plants. Int. J. Pharm. Pharm. Sci. 2015, 7, 123–128. [Google Scholar]
- Es’haghi, Z. Photodiode array detection in clinical applications; Quantitative analyte assay advantages, limitations and disadvantages. Photodiodes-Commun. Bio-Sens. Meas. High-Energy Phys. 2011, 10, 161–182. [Google Scholar]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. In Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 579–605. [Google Scholar]
- Anderson, G. Phytochemicals. Dyn. Chiropr. 2004, 2, 5. [Google Scholar]
- Atmani, D.; Chaher, N.; Atmani, D.; Berboucha, M.; Debbache, N.; Boudaoud, H. Flavonoids in human health: From structure to biological activity. Curr. Nutr. Food Sci. 2009, 5, 225–237. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Ragab, F.; Yahya, T.; El-Naa, M.; Arafa, R. Design, synthesis and structure–activity relationship of novel semi-synthetic flavonoids as antiproliferative agents. Eur. J. Med. Chem. 2014, 82, 506–520. [Google Scholar] [CrossRef]
- Dubbu, S.; Chennaiah, A.; Verma, A.K.; Vankar, Y.D. Stereoselective synthesis of 2-deoxy-β-C-aryl/alkyl glycosides using Prins cyclization: Application in the synthesis of C-disaccharides and differently protected C-aryl glycosides. Carbohydr. Res. 2018, 468, 64–68. [Google Scholar] [CrossRef]
- Van Acker, S.A.; de Groot, M.J.; van den Berg, D.J.; Tromp, M.N.; Donné-Op den Kelder, G.; van der Vijgh, W.J.; Bast, A. A quantum chemical explanation of the antioxidant activity of flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharmacal Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, J.-Y.; Lee, J.-Y.; Kang, C.-M.; Kwon, H.-J.; Yoo, Y.-D.; Kim, T.-W.; Lee, Y.-S.; Lee, S.-J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Scambia, G.; Fattorossi, A.; Capelli, A.; Piantelli, M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef]
- Kim, J.-E.; Lee, D.-E.; Lee, K.W.; Son, J.E.; Seo, S.K.; Li, J.; Jung, S.K.; Heo, Y.-S.; Mottamal, M.; Bode, A.M.; et al. Isorhamnetin Suppresses Skin Cancer through Direct Inhibition of MEK1 and PI3-K. Cancer Prev. Res. 2011, 4, 582–591. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.M.; Kwon, B.M.; Baek, N.I.; Kim, S.H.; Chung, I.S.; Park, M.H.; Park, H.W.; Lee, J.H.; Park, H.W.; Kim, E.J.; et al. Inhibitory activity of isorhamnetin from Persicaria thunbergii on Farnesyl Protein Transferase. Arch. Pharm. Res. 2005, 28, 169–171. [Google Scholar] [CrossRef]
- Jaramillo, S.; Lopez, S.; Varela, L.M.; Rodriguez-Arcos, R.; Jimenez, A.; Abia, R.; Guillen, R.; Muriana, F.J. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J. Agric. Food Chem. 2010, 58, 10869–10875. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Zhao, X.; Luo, F.; Li, X.; Zhu, H.; Sun, C.; Chen, K. Chemopreventive effect of flavonoids from Ougan (Citrus reticulata cv. Suavissima) fruit against cancer cell proliferation and migration. J. Funct. Foods 2014, 10, 511–519. [Google Scholar] [CrossRef]
- Horváthová, K.; Novotný, L.; Tóthová, D.; Vachálková, A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma 2004, 51, 395–399. [Google Scholar] [PubMed]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef] [PubMed]
- Rusak, G.; Gutzeit, H.O.; Müller, J.L. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr. Res. 2005, 25, 143–155. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Namgoong, S.Y.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of naturally occurring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 1994, 54, 313–320. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [Green Version]
- Kothan, S.; Dechsupa, S.; Leger, G.; Moretti, J.L.; Vergote, J.; Mankhetkorn, S. Spontaneous mitochondrial membrane potential change during apoptotic induction by quercetin in K562 and K562/adr cells. Can. J. Physiol. Pharmacol. 2004, 82, 1084–1090. [Google Scholar] [CrossRef]
- Iori, F.; da Fonseca, R.; Ramos, M.J.; Menziani, M.C. Theoretical quantitative structure-activity relationships of flavone ligands interacting with cytochrome P450 1A1 and 1A2 isozymes. Bioorg. Med. Chem. 2005, 13, 4366–4374. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Sheikhpour, E.; Noorbakhsh, P.; Foroughi, E.; Farahnak, S.; Nasiri, R.; Neamatzadeh, H. A survey on the role of interleukin-10 in breast cancer: A Narrative. Rep. Biochem. Mol. Biol. 2018, 7, 30–37. [Google Scholar]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Cumella, J.; Faden, H.; Middleton, E. Selective activity of plant flavonoids on neutrophil chemiluminescence (CL). J. Allergy Clin. Immunol. 1987, 79, 157. [Google Scholar]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Isoda, H.; Motojima, H.; Onaga, S.; Samet, I.; Villareal, M.O.; Han, J. Analysis of the erythroid differentiation effect of flavonoid apigenin on K562 human chronic leukemia cells. Chem.-Biol. Interact. 2014, 220, 269–277. [Google Scholar] [CrossRef] [Green Version]





| Sample | EC50 (µM) * | TEAC (mM) * |
|---|---|---|
| RT | 386.0 ± 21.4 a | 18.8 ± 4.4 a |
| QT | 78.5 ± 4.8 b | 191.5 ± 15.1 b |
| IRR | 1647.0 ± 109.7 c | 5.94 ± 0.5 c |
| IR | 222.3± 17.0 d | 68.9 ± 7.3 d |
| Sample | Additional Sample | CI |
|---|---|---|
| QT | RT | 1.13 |
| IR | 0.62 | |
| RT | QT | 1.05 |
| IRR | 0.82 | |
| IR | IRR | 1.32 |
| QT | 0.85 | |
| IRR | IR | 0.88 |
| QT | 1.56 |
| Cancer | Cell Type | IC50 (µM) * | |||
|---|---|---|---|---|---|
| RT | QT | IRR | IR | ||
| Leukemia | EoL-1 | >200.0 a | 6.0 ± 0.1 b | 118.3 ± 20.6 c | 5.3 ± 0.1 d |
| MV4-11 | >200.0 a | 20.4 ± 6.6 b | >200.0 a | 5.9 ± 1.7 c | |
| Molt4 | >200.0 a | 155.3 ± 107.4 a | >200.0 a | >300.0 a | |
| U937 | >200.0 a | 28.8 ± 7.8 b | >200.0 a | >300.0 a | |
| K562 | >200.0 a | 28.7 ± 3.6 b | >200.0 a | 45.9 ± 11.4 c | |
| K562/ADR | >200.0 a | 20.9 ± 3.0 b | >200.0 a | 25.1 ± 7.3 b | |
| Cervical carcinoma | KB-3-1 | >200.0 a | >300.0 a | >200.0 a | 67.5 ± 23.2 b |
| Breast cancer | MCF-7 | >200.0 a | >300.0 a | >200.0 a | >300.0 a |
| Cell Type | Sample | Additional Sample | CI |
|---|---|---|---|
| K562 | QT | IR | 0.68 |
| IR | QT | 1.14 | |
| K562/ADR | QT | IR | 0.92 |
| IR | QT | 1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naksuriya, O.; Daowtak, K.; Tima, S.; Okonogi, S.; Mueller, M.; Toegel, S.; Khonkarn, R. Hydrolyzed Flavonoids from Cyrtosperma johnstonii with Superior Antioxidant, Antiproliferative, and Anti-Inflammatory Potential for Cancer Prevention. Molecules 2022, 27, 3226. https://doi.org/10.3390/molecules27103226
Naksuriya O, Daowtak K, Tima S, Okonogi S, Mueller M, Toegel S, Khonkarn R. Hydrolyzed Flavonoids from Cyrtosperma johnstonii with Superior Antioxidant, Antiproliferative, and Anti-Inflammatory Potential for Cancer Prevention. Molecules. 2022; 27(10):3226. https://doi.org/10.3390/molecules27103226
Chicago/Turabian StyleNaksuriya, Ornchuma, Krai Daowtak, Singkome Tima, Siriporn Okonogi, Monika Mueller, Stefan Toegel, and Ruttiros Khonkarn. 2022. "Hydrolyzed Flavonoids from Cyrtosperma johnstonii with Superior Antioxidant, Antiproliferative, and Anti-Inflammatory Potential for Cancer Prevention" Molecules 27, no. 10: 3226. https://doi.org/10.3390/molecules27103226