Improved Hydrophobicity of Macroalgae Biopolymer Film Incorporated with Kenaf Derived CNF Using Silane Coupling Agent
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Isolation and Characterisation of CNF via Supercritical CO2
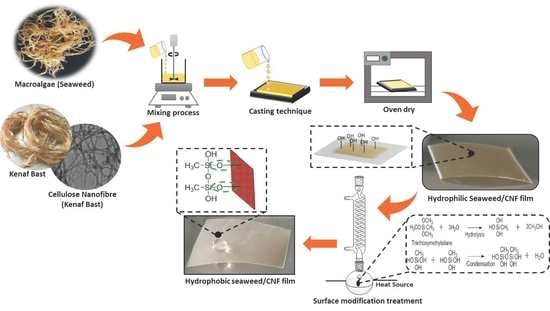
2.3. Preparation of Macroalgae/CNF Biopolymer Film
2.4. Surface Treatment of Macroalgae Film Using Silane
2.5. Characterisation of Macroalgae Biopolymer Films
3. Results and Discussion
3.1. Characterisation of Macroalgae/CNF Biopolymer Film
3.2. Morphology of the Unmodified and Modified Macroalgae/CNF Biopolymer Film
3.3. Mechanical Properties of Macroalgae/CNF Biofilm
3.4. Wettability of Macroalgae Kenaf Bast CNF Biopolymer Film
3.5. Thermal Behaviour of Macroalgae Biopolymer Films
3.6. Mechanism of CNF Macroalgae Film Surface Treatment Using Triethoxymethylsilane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Palmer, J.L.; Morris, L.A.; Ryu, M.Y.; Wickiser, J.K. Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. Molecules 2018, 23, 1405. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterisation, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.; Saurabh, C.; Leh, C.; Lai, T.; Chong, E.; Fazita, M.; Hafiidz, J.M.; Banerjee, A.; Syakir, M. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Hermawan, D.; Lai, T.K.; Jafarzadeh, S.; Gopakumar, D.A.; Hasan, M.; Owolabi, F.T.; Aprilia, N.S.; Rizal, S.; Abdul Khalil, H.P.S. Development of seaweed-based bamboo microcrystalline cellulose films intended for sustainable food packaging applications. Bioresource 2019, 14, 3389–3410. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.; Lai, T.; Easa, A.; Rosamah, E.; Fazita, M.; Syakir, M.; Adnan, A.; Fizree, H. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Rizal, S.; Lai, T.K.; Muksin, U.; Olaiya, N.G.; Abdullah, C.K.; Ikramullah; Yahya, E.B.; Chong, E.W.N.; Abdul Khalil, H.P.S. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers 2020, 12, 2554. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Wang, L.-F. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydr. Polym. 2013, 96, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved mechanical properties of k-carrageenan-based nanocomposite films reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef]
- Balea, A.; Sanchez-Salvador, J.L.; Monte, M.C.; Merayo, N.; Negro, C.; Blanco, A. In situ production and application of cellulose nanofibers to improve recycled paper production. Molecules 2019, 24, 1800. [Google Scholar] [CrossRef]
- Sharip, N.S.; Ariffin, H.; Andou, Y.; Shirosaki, Y.; Bahrin, E.K.; Jawaid, M.; Tahir, P.M.; Ibrahim, N.A. Process Optimisation of Ultra-High Molecular Weight Polyethylene/Cellulose Nanofiber Bionanocomposites in Triple Screw Kneading Extruder by Response Surface Methodology. Molecules 2020, 25, 4498. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.-Q.; Gustafsson, S.; Strømme, M.; Mihranyan, A.; Lindh, J. Cellulose nanofibers prepared via pre-treatment based on Oxone® oxidation. Molecules 2017, 22, 2177. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, B.; Lu, M.; Li, Z.; Qiang, H. Fabrication and Performance of Self-Supported Flexible Cellulose Nanofibrils/Reduced Graphene Oxide Supercapacitor Electrode Materials. Molecules 2020, 25, 2793. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Yusra, A.I.; Bhat, A.; Jawaid, M. Cell wall ultrastructure, anatomy, lignin distribution, and chemical composition of Malaysian cultivated kenaf fiber. Ind. Crops Prod. 2010, 31, 113–121. [Google Scholar] [CrossRef]
- Anuar, H.; Zuraida, A. Improvement in mechanical properties of reinforced thermoplastic elastomer composite with kenaf bast fibre. Compos. Part B Eng. 2011, 42, 462–465. [Google Scholar] [CrossRef]
- Sulaiman, S.; Sahat, N.S.; Omar, F.N.; Mokhtar, M.N.; Naim, M.N.; Baharuddin, A.S.; Salleh, M.A.M. Chemical-Physical Treatment for Production of Cellulose Nanofiber from Kenaf Bast Fiber. J. Nat. Fibers 2020, 1–12. [Google Scholar] [CrossRef]
- Rizal, S.; Sadasivuni, K.K.; Atiqah, M.N.; Olaiya, N.G.; Paridah, M.T.; Abdullah, C.; Alfatah, T.; Mistar, E.; Abdul Khalil, H.P.S. The role of cellulose nanofibrillated fibers produced with combined supercritical carbon dioxide and high-pressure homogenisation process as reinforcement material in biodegradable polymer. Polym. Compos. 2021, 42, 1795–1808. [Google Scholar] [CrossRef]
- Hemmati, F.; Farizeh, T.; Mohammadi-Roshandeh, J. Lignocellulosic Fiber-Reinforced PLA Green Composites: Effects of Chemical Fiber Treatment. In Biocomposite Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 97–204. [Google Scholar]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterisation of starch-based composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef]
- Granda, L.A.; Oliver-Ortega, H.; Fabra, M.J.; Tarrés, Q.; Pèlach, M.À.; Lagarón, J.M.; Méndez, J.A. Improved process to obtain nanofibrillated cellulose (CNF) reinforced starch films with upgraded mechanical properties and barrier character. Polymers 2020, 12, 1071. [Google Scholar] [CrossRef]
- Thakur, M.K.; Gupta, R.K.; Thakur, V.K. Surface modification of cellulose using silane coupling agent. Carbohydr. Polym. 2014, 111, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.d.S.; Panthapulakkal, S.; Konar, S.K.; Sain, M.; Bufalinof, L.; Raabe, J.; Miranda, I.P.d.A.; Martins, M.A.; Tonoli, G.H.D. Improving cellulose nanofibrillation of non-wood fiber using alkaline and bleaching pre-treatments. Ind. Crops Prod. 2019, 131, 203–212. [Google Scholar] [CrossRef]
- Morita, A.; Matsuba, G.; Fujimoto, M. Evaluation of hydrophilic cellulose nanofiber dispersions in a hydrophobic isotactic polypropylene composite. J. Appl. Polym. Sci. 2021, 138, 49896. [Google Scholar] [CrossRef]
- Fattahi, R.; Seyedain–Ardabili, M. A comparative study on the effect of homogenisation conditions on the properties of the film–forming emulsions and the resultant films. Food Chem. 2021, 352, 129319. [Google Scholar] [CrossRef]
- Atiqah, M.; Gopakumar, D.A.; FAT, O.; Pottathara, Y.B.; Rizal, S.; Aprilia, N.; Hermawan, D.; Paridah, M.; Thomas, S.; Abdul Khalil, H.P.S. AK Extraction of cellulose nanofibers via eco-friendly supercritical carbon dioxide treatment followed by mild acid hydrolysis and the fabrication of cellulose nanopapers. Polymers 2019, 11, 1813. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofibre loading and plasticiser content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef]
- Al, G.; Aydemir, D.; Kaygin, B.; Ayrilmis, N.; Gunduz, G. Preparation and characterisation of biopolymer nanocomposites from cellulose nanofibrils and nanoclays. J. Compos. Mater. 2018, 52, 689–700. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Burón, M.; Gude, M.R.; Chaos-Morán, R.; Campo, M.; Ureña, A. Effects of dispersion techniques of carbon nanofibers on the thermo-physical properties of epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 2722–2730. [Google Scholar] [CrossRef]
- Doh, H.; Dunno, K.D.; Whiteside, W.S. Preparation of novel seaweed nanocomposite film from brown seaweeds Laminaria japonica and Sargassum natans. Food Hydrocoll. 2020, 105, 105744. [Google Scholar] [CrossRef]
- Li, J.; Jiang, S.; Wei, Y.; Li, X.; Shi, S.Q.; Zhang, W.; Li, J. Facile fabrication of tough, strong, and biodegradable soy protein-based composite films with excellent UV-blocking performance. Compos. Part B Eng. 2021, 211, 108645. [Google Scholar] [CrossRef]
- Salehudin, M.; Salleh, E.; Muhamad, I.I.; Mamat, S. Starch-based biofilm reinforced with empty fruit bunch cellulose nanofibre. Mater. Res. Innov. 2014, 18 (Suppl. 6), 322–325. [Google Scholar] [CrossRef]
- Agbahoungbata, M.Y.; Fatombi, J.K.; Ayedoun, M.A.; Idohou, E.; Sagbo, E.V.; Osseni, S.A.; Mama, D.; Aminou, T. Removal of reactive dyes from their aqueous solutions using Moringa oleifera seeds and Grewia venusta peel. Desalination Water Treat. 2016, 57, 22609–22617. [Google Scholar] [CrossRef]
- Affes, S.; Maalej, H.; Aranaz, I.; Kchaou, H.; Acosta, N.; Heras, Á.; Nasri, M. Controlled size green synthesis of bioactive silver nanoparticles assisted by chitosan and its derivatives and their application in biofilm preparation. Carbohydr. Polym. 2020, 236, 116063. [Google Scholar] [CrossRef] [PubMed]
- Garrido, T.; Etxabide, A.; Guerrero, P.; De la Caba, K. Characterization of agar/soy protein biocomposite films: Effect of agar on the extruded pellets and compression moulded films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, H.; Ji, Y.; Zhang, Y. Functionally modified monodisperse core–shell silica nanoparticles: Silane coupling agent as capping and size tuning agent. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 411, 111–121. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, P.; Wang, M.; Zhao, H.; Yang, J.; Xu, F. Sustainable, reusable, and superhydrophobic aerogels from microfibrillated cellulose for highly effective oil/water separation. ACS Sustain. Chem. Eng. 2016, 4, 6409–6416. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyekanmi, A.A.; Saharudin, N.I.; Hazwan, C.M.; H. P. S., A.K.; Olaiya, N.G.; Abdullah, C.K.; Alfatah, T.; Gopakumar, D.A.; Pasquini, D. Improved Hydrophobicity of Macroalgae Biopolymer Film Incorporated with Kenaf Derived CNF Using Silane Coupling Agent. Molecules 2021, 26, 2254. https://doi.org/10.3390/molecules26082254
Oyekanmi AA, Saharudin NI, Hazwan CM, H. P. S. AK, Olaiya NG, Abdullah CK, Alfatah T, Gopakumar DA, Pasquini D. Improved Hydrophobicity of Macroalgae Biopolymer Film Incorporated with Kenaf Derived CNF Using Silane Coupling Agent. Molecules. 2021; 26(8):2254. https://doi.org/10.3390/molecules26082254
Chicago/Turabian StyleOyekanmi, Adeleke A., N. I. Saharudin, Che Mohamad Hazwan, Abdul Khalil H. P. S., Niyi G. Olaiya, Che K. Abdullah, Tata Alfatah, Deepu A. Gopakumar, and Daniel Pasquini. 2021. "Improved Hydrophobicity of Macroalgae Biopolymer Film Incorporated with Kenaf Derived CNF Using Silane Coupling Agent" Molecules 26, no. 8: 2254. https://doi.org/10.3390/molecules26082254