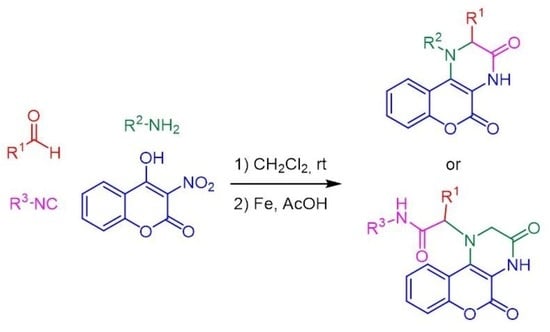
3.4.1. Three-Component Condensation
Our previously reported procedure [
51] was followed. Briefly, isocyanide (
14, 0.5 mmol) and enol (
15, 0.5 mmol) were successively added to a solution of imine (
16, 0.5 mmol) in CH
2Cl
2 (1 mL), and the resulting mixture was stirred at 20 °C for 3 h. Removal of the solvent and purification by column chromatography (SiO
2, gradient from 100% hexanes to hexanes–EtOAc, 7:3) gave the corresponding enamines
17a–
k (
Table 1).
2-(Benzyl(3-nitro-2-oxo-2H-chromen-4-yl)amino)-2-(2-bromophenyl)-N-cyclohexyl acetamide (17b). Obtained from isocyanide 14a, enol 15 and imine 16b, from isocyanide 14a, enol 15 and imine 16b, as a yellow solid (260 mg, 88%); m.p. 165–167 °C; IR (cm−1) 3341, 3064, 2933, 2854, 1714, 1677, 1600, 1549, 1465, 1350, 1278, 1116, 1054, 925, 760; 1H-NMR (500 MHz, CDCl3) δ 8.14 (d, J = 8.0 Hz, 1H), 7.63–7.57 (m, 3H), 7.42 (dt, J = 7.5, 0.9 Hz, 1H), 7.40 (dt, J = 3.7, 1.0 Hz, 1H), 7.38–7.36 (m, 1H), 7.31 (dd, J = 8.3, 0.8 Hz, 1H), 7.28 (dd, J = 7.8, 1.4 Hz, 1H), 7.25–7.18 (m, 5H), 5.87 (s, 1H), 5.56 (d, J = 8.0 Hz, 1H), 4.77 (d, J = 14.8 Hz, 1H), 3.99 (d, J = 14.8 Hz, 1H), 3.82–3.73 (m, 1H), 1.93–1.54 (m, 5H), 1.39–0.97 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 167.45 (C), 155.12 (C), 153.00 (C), 152.48 (C), 135.14 (C), 134.33 (C), 133.96 (CH), 133.82 (CH), 130.99 (CH), 130.13 (CH), 129.02 (CH), 128.61 (CH), 128.44 (CH), 128.24 (CH), 127.49 (CH), 126.33 (C), 125.12 (CH), 118.45 (C), 117.90 (CH), 68.92 (CH), 54.76 (CH2), 49.26 (CH), 32.73 (CH2), 32.71 (CH2), 24.82 (CH2), 24.74 (CH2); MS (qTOF) m/z (%) 592 (M+ + 3, 28), 590 (M+ + 1, 30), 574 (42), 572 (44), 510 (98), 508 (100); HRMS (qTOF) Calcd for C30H29BrN3O5: 590.1296. Found: 590.1285.
2-(Benzyl(3-nitro-2-oxo-2H-chromen-4-yl)amino)-N-cyclohexyl-2-(p-tolyl)acetamide (17c). Obtained from isocyanide 14a, enol 15 and imine 16c, as a yellow solid (208 mg, 79%); m.p. 141–143 °C; IR (cm−1) 3369, 2928, 2853, 1729, 1681, 1601, 1549, 1451, 1403, 1350, 1116, 1054, 791; 1H-NMR (500 MHz, CDCl3) δ 8.04 (d, J = 8.1 Hz, 1H), 7.56 (dt, J = 7.3, 1.4 Hz, 1H), 7.31–7.26 (m, 2H), 7.24–7.11 (m, 9H), 5.78 (d, J = 8.1 Hz, 1H), 5.16 (s, 1H), 4.64 (d, J = 14.9 Hz, 1H), 4.19 (d, J = 14.9 Hz, 1H), 3.76–3.67 (m, 1H), 2.33 (s, 3H), 1.87–1.53 (m, 5H), 1.36–0.95 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 168.30 (C), 155.08 (C), 153.26 (C), 152.49 (C), 139.50 (C), 135.31 (C), 133.89 (CH), 131.78 (C), 129.90 (CH), 129.16 (CH), 129.04 (CH), 128.57 (C), 128.45 (CH), 128.39 (CH), 124.93 (CH), 118.38 (C), 117.65 (CH), 71.15 (CH), 55.96 (CH2), 48.99 (CH), 32.79 (CH2), 32.70 (CH2), 27.07 (CH2), 24.87 (CH2), 24.79 (CH2), 21.32 (CH3); MS (qTOF) m/z (%) 526 (M+ + 1, <5), 479 (10), 347 (100), 146 (54); HRMS (qTOF) Calcd for C33H32N3O5: 526.2326. Found: 526.2326.
2-(Benzyl(3-nitro-2-oxo-2H-chromen-4-yl)amino)-N-cyclohexyl-2-(4-(trifluoromethyl) phenyl)acetamide (17d). Obtained from isocyanide 14a, enol 15 and imine 16d, as a yellow solid (226 mg, 78%), m.p. 135–137 °C; IR (cm−1) 3365, 2932, 2855, 1730, 1684, 1603, 1550, 1324, 1168, 1127, 1068, 761, 699; 1H-NMR (500 MHz, CDCl3) δ 7.90 (d, J = 7.3 Hz, 1H), 7.61–7.57 (m, 3H), 7.52 (d, J = 8.2 Hz, 2H), 7.30 (d, J = 7.7 Hz, 2H), 7.24–7.18 (m, 3H), 7.10 (dd, J = 6.5, 1.6 Hz, 2H), 5.88 (d, J = 8.1 Hz, 1H), 5.16 (s, 1H), 4.63 (d, J = 14.7 Hz, 1H), 4.23 (d, J = 14.7 Hz, 1H), 3.71–3.61 (m, 1H), 1.84–1.55 (m, 5H), 1.35–0.93 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 167.30 (C), 154.79 (C), 153.12 (C), 152.48 (C), 139.01 (C), 134.71 (C), 134.23 (CH), 131.76 (C), 131.50 (C), 129.35 (CH), 129.29 (CH), 128.75 (CH), 128.70 (CH), 128.28 (CH), 126.15 (CH), 126.12 (CH), 125.05 (CH), 122.73 (C), 118.21 (C), 117.80 (CH), 70.56 (CH), 56.77 (CH2), 49.15 (CH), 32.68 (CH2), 32.60 (CH2), 25.41 (CH2), 24.82 (CH2), 24.75 (CH2); MS (qTOF) m/z (%) 580 (M+ + 1, 100), 391 (13), 309 (28); HRMS (qTOF) Calcd for C31H29F3N3O5: 580.2059. Found: 580.2059.
2-((Benzo[d][1,3]dioxol-5-ylmethyl)(3-nitro-2-oxo-2H-chromen-4-yl)amino)-N-cyclohexyl-2-phenylacetamide (17e). Obtained from isocyanide 14a, enol 15 and imine 16e, as a yellow solid (217 mg, 78%); m.p. 135–136 °C; IR (cm−1) 3369, 2930, 2853, 1728, 1681, 1601, 1549, 1504, 1489, 1446, 1401, 1347, 1249, 929, 761; 1H-NMR (500 MHz, CDCl3) δ 8.07 (dd, J = 8.1, 1.3 Hz, 1H), 7.56 (dt, J = 6.9, 1.4 Hz, 1H), 7.38–7.28 (m, 7H), 6.74 (d, J = 1.4 Hz, 1H), 6.61 (d, J = 7.9, Hz, 1H), 6.57 (dd, J = 8.0, 1.5 Hz, 1H), 5.89 (s, 2H), 5.78 (d, J = 8.1 Hz, 1H), 5.19 (s, 1H), 4.56 (d, J = 14.8 Hz, 1H), 4.09 (d, J = 14.8 Hz, 1H), 3.76–3.67 (m, 1H), 1.89–1.53 (m, 5H), 1.37–0.95 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 168.11 (C), 155.06 (C), 152.50 (C), 147.83 (C), 147.73 (C), 134.78 (C), 134.00 (CH), 129.52 (CH), 129.23 (CH), 129.12 (CH), 128.91 (C), 128.31 (CH), 125.06 (CH), 122.90 (CH), 118.25 (C), 117.71 (CH), 109.38 (CH), 108.16 (CH), 101.22 (CH2), 71.29 (CH), 55.71 (CH2), 49.04 (CH), 32.76 (CH2), 32.67 (CH2), 27.05 (CH2), 24.85 (CH2), 24.77 (CH2); MS (qTOF) m/z (%) 556 (M+ + 1, 672), 457 (20), 353 (100); HRMS (qTOF) Calcd for C31H30N3O7: 556.2084. Found: 556.2089.
2-((Benzo[d][1,3]dioxol-5-ylmethyl)(3-nitro-2-oxo-2H-chromen-4-yl)amino)-N-cyclohexyl-2-(p-tolyl)acetamide (17f). Obtained from isocyanide 14a, enol 15 and imine 16f, as a yellow solid (202 mg, 71%); m.p. 145–146 °C; IR (cm−1) 3424, 2929, 2853, 1728, 1679, 1601, 1549, 1489, 1446, 1249, 1039, 929, 761; 1H-NMR (500 MHz, CDCl3) δ 8.09 (d, J = 7.2 Hz, 1H), 7.58 (dt, J = 6.4, 1.3 Hz, 1H), 7.35–7.27 (m, 2H), 7.21 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 7.9 Hz, 2H), 6.75 (s, 1H), 6.63–6.55 (m, 2H), 5.88 (s, 2H), 5.72 (d, J = 8.1 Hz, 1H), 5.16 (s, 1H), 4.55 (d, J = 14.8 Hz, 1H), 4.08 (d, J = 15.0 Hz, 1H), 3.77–3.68 (m, 1H), 2.33 (s, 3H), 1.88–1.54 (m, 5H), 1.35–0.95 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 168.36 (C), 153.15 (C), 152.55 (C), 147.84 (C), 147.71 (C), 139.54 (C), 133.92 (CH), 131.77 (C), 130.54 (C), 129.93 (CH), 129.07 (CH), 128.36 (CH), 125.02 (CH), 122.90 (CH), 118.36 (C), 118.32 (C), 117.69 (CH), 109.43 (CH), 108.15 (CH), 101.20 (CH2), 71.14 (CH), 55.58 (CH2), 49.04 (CH), 32.82 (CH2), 32.73 (CH2), 27.07 (CH2), 24.88 (CH2), 24.80 (CH2), 21.32 (CH3); MS (qTOF) m/z (%) 570 (M+ + 1, 70), 353 (100); HRMS (qTOF) Calcd for C32H32N3O7: 570.2240. Found: 570.2236.
2-((Benzo[d][1,3]dioxol-5-ylmethyl)(3-nitro-2-oxo-2H-chromen-4-yl)amino)-N-cyclohexyl-2-(4-(trifluoromethyl)phenyl)acetamide (17g). Obtained from isocyanide 14a, enol 15 and imine 16g, as a yellow solid (250 mg, 80%); m.p. 129–131 °C; IR (cm−1) 3362, 2931, 2854, 1727, 1683, 1603, 1550, 1490, 1447, 1324, 1250, 1167, 1127, 1068, 930, 761; 1H-NMR (500 MHz, CDCl3) δ 7.96 (dd, J = 8.1, 1.1 Hz, 1H), 7.64–7.56 (m, 3H), 7.52 (d, J = 8.2 Hz, 2H), 7.35–7.29 (m, 2H), 6.67 (d, J = 1.5 Hz, 1H), 6.62 (d, J = 7.9 Hz, 1H), 6.52 (dd, J = 8.0, 1.5 Hz, 1H), 5.90 (s, 2H), 5.82 (d, J = 8.0 Hz, 1H), 5.16 (s, 1H), 4.55 (d, J = 14.6 Hz, 1H), 4.16 (d, J = 14.6 Hz, 1H), 3.72–3.63 (m, 1H), 1.87–1.52 (m, 5H), 1.35–0.94 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 167.34 (C), 154.78 (C), 153.00 (C), 152.53 (C), 148.00 (C), 147.96 (C), 138.97 (C), 134.28 (CH), 129.35 (CH), 128.39 (C), 128.20 (CH), 126.17 (CH), 126.14 (CH), 125.14 (CH), 123.07 (CH), 118.16 (C), 117.85 (CH), 109.48 (CH), 108.28 (CH), 101.34 (CH2), 70.52 (CH), 56.42 (CH2), 49.19 (CH), 32.71 (CH2), 32.64 (CH2), 27.07 (CH2), 24.83 (CH2), 24.76 (CH2); MS (qTOF) m/z (%) 624 (M+ + 1, 35), 353 (100); HRMS (qTOF) Calcd for C32H29F3N3O7: 624.1958. Found: 624.1942.
N-Cyclohexyl-2-(cyclohexyl(3-nitro-2-oxo-2H-chromen-4-yl)amino)-2-(p-tolyl)acetamide (17h). Obtained from isocyanide 14a, enol 15 and imine 16h, as a pale orange solid (205 mg, 79%); m.p. 68–70 °C; IR (cm−1) 3415, 2931, 2855, 1736, 1662, 1605, 1540, 1451, 1374, 1276, 1111, 1055, 762; 1H-NMR (500 MHz, CDCl3) δ 7.98 (bs, 1H), 7.53 (dt, J = 7.2, 1.3 Hz, 1H), 7.32–7.28 (m, 3H), 7.21 (d, J = 7.6 Hz, 1H), 6.90 (d, J = 8.0 Hz, 2H), 6.53 (d, J = 5.8 Hz, 1H), 5.07 (s, 1H), 3.72–3.61 (m, 1H), 3.32 (tt, J = 11.6, 3.4 Hz, 1H), 2.17 (s, 3H), 2.10–0.81 (m, 20H); 13C-NMR (126 MHz, CDCl3) δ 170.63 (C), 154.63 (C), 153.53 (C), 151.63 (C), 138.79 (C), 133.82 (CH), 132.54 (C), 129.27 (CH), 129.21 (CH), 129.12 (CH), 128.70 (C), 127.78 (CH), 127.12 (CH), 124.57 (CH), 120.12 (C), 117.24 (CH), 72.17 (CH), 66.58 (CH), 48.60 (CH), 32.73 (CH2), 32.63 (CH2), 32.45 (CH2), 32.28 (CH2), 27.06 (CH2), 26.09 (CH2), 26.08 (CH2), 24.97 (CH2), 24.94 (CH2), 21.14 (CH3); MS (qTOF) m/z (%) 518 (M+ + 1, 30), 517 (35), 447 (100); HRMS (qTOF) Calcd for C30H36N3O5: 518.2655. Found: 518.2648.
N-Cyclohexyl-2-(cyclohexyl(3-nitro-2-oxo-2H-chromen-4-yl)amino)-2-(4-(trifluoromethyl)phenyl)acetamide (17i). Obtained from isocyanide 14a, enol 15 and imine 16i, as a pale orange solid (243 mg, 85%); m.p. 133–135 °C; IR (cm−1) 3386, 2933, 2856, 1737, 1681, 1606, 1541, 1325, 1167, 1127, 1068, 762; 1H-NMR (500 MHz, CDCl3) δ 7.93 (bs, 1H), 7.59 (d, J = 8.2 Hz, 2H), 7.54 (t, J = 7.3 Hz, 1H), 7.40 (d, J = 8.2 Hz, 2H), 7.32 (t, J = 7.7 Hz, 1H), 7.24 (d, J = 8.3 Hz, 1H), 6.55 (bs, 1H), 5.16 (s, 1H), 3.68–3.57 (m, 1H), 3.30 (tt, J = 11.6, 3.4 Hz, 1H), 2.09–1.53 (m, 10H), 1.37–0.95 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 169.44 (C), 154.32 (C), 152.78 (C), 151.79 (C), 140.01 (C), 134.25 (CH), 128.81 (CH), 125.58 (CH), 125.55 (CH), 124.86 (CH), 119.84 (C), 117.56 (CH), 72.08 (CH), 66.63 (CH), 48.84 (CH), 32.66 (CH2), 32.58 (CH2), 32.49 (CH2), 32.30 (CH2), 27.07 (CH2), 26.07 (CH2), 25.47 (CH2), 25.23 (CH2), 24.92 (CH2), 24.89 (CH2); MS (qTOF) m/z (%) 572 (M+ + 1, 69), 473 (100), 383 (17), 301 (171); HRMS (qTOF) Calcd for C30H33F3N3O5: 572.2372. Found: 572.2367.
N-Cyclohexyl-2-(3,4-dimethoxyphenyl)-2-((3-nitro-2-oxo-2H-chromen-4-yl)(phenyl)amino) acetamide (17k). Obtained from isocyanide 14a, enol 15 and imine 16k, as an orange solid (112 mg, 40%); m.p. 129–130 °C; IR (cm−1) 3403, 2931, 2853, 1740, 1681, 1604, 1517, 1451, 1373, 1265, 1148, 761; 1H-NMR (500 MHz, CDCl3) δ 7.67 (d, J = 7.0 Hz, 1H), 7.55 (dt, J = 6.1, 1.5 Hz, 1H), 7.29–7.20 (m, 5H), 7.03 (t, J = 7.4 Hz, 1H), 6.99 (d, J = 8.0 Hz, 2H), 6.76 (dd, J = 8.3, 2.1 Hz, 2H), 6.67 (d, J = 1.9 Hz, 2H), 6.61 (d, J = 8.4 Hz, 2H), 6.37 (d, J = 8.2 Hz, 2H), 5.50 (s, 2H), 3.87–3.78 (m, 2H), 3.77 (s, 3H), 3.58 (s, 3H), 1.94–1.52 (m, 5H), 1.39–0.94 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 168.15 (C), 153.89 (C), 152.90 (C), 150.26 (C), 149.83 (C), 148.68 (C), 144.87 (C), 134.24 (CH), 129.75 (CH), 128.78 (CH), 125.29 (CH), 124.44 (C), 123.13 (CH), 123.04 (CH), 118.23 (C), 117.81 (CH), 117.60 (CH), 112.72 (CH), 110.84 (CH), 69.97 (CH), 55.90 (CH3), 55.65 (CH3), 48.64 (CH), 32.83 (CH2), 32.43 (CH2), 25.43 (CH2), 24.71 (CH2), 24.58 (CH2); MS (qTOF) m/z (%) 558 (M+ + 1, <5), 478 (35), 328 (57); HRMS (qTOF) Calcd for C31H32N3O7: 558.2240. Found: 558.2234.
3.4.2. Four-Component Condensation
Amine
13 (0.5 mmol) was added to a solution of aldehyde
12 (0.5 mmol) in of dry acetonitrile (1 mL). The resulting mixture was stirred for 15 min at rt and then isocyanide
14 (0.5 mmol) and enol
15 (0.5 mmol) were successively added. After 4 days stirring at room temperature, the reaction went to completion, as judged by tlc. Then 10% HCl (2 mL) was added, the mixture was washed with H
2O (15 mL), extracted with CH
2Cl
2 (3 × 20 mL) and dried over Na
2SO
4. Removal of the solvent and purification by column chromatography (SiO
2, gradient from 100% hexanes to hexanes–EtOAc, 7:3) gave the corresponding enamines
17l–s (
Table 2).
Methyl N-(2-(cyclohexylamino)-2-oxo-1-phenylethyl)-N-(3-nitro-2-oxo-2H-chromen-4-yl)glycinate (17l). Obtained from aldehyde 12a, amine 13e, isocyanide 14a and enol 15, as a pale orange solid (141 mg, 57%); m.p. 132–134 °C; IR (cm−1) 3359, 2931, 2854, 1730, 1681, 1606, 1554, 1452, 1212, 760; 1H-NMR (500 MHz, CDCl3) δ 8.12 (d, J = 8.1 Hz, 1H), 7.63 (t, J = 7.7 Hz, 1H), 7.44–7.33 (m, 5H), 5.90 (d, J = 8.0 Hz, 1H), 5.39 (s, 1H), 4.10 (d, J = 18.0 Hz, 1H), 3.80 (d, J = 18.0 Hz, 1H), 3.74–3.66 (m, 1H), 3.64 (s, 3H), 1.91–0.93 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 169.12 (C), 167.52 (C), 152.60 (C), 152.42 (C), 134.39 (C), 133.94 (CH), 129.57 (CH), 129.24 (CH), 128.91 (CH), 127.89 (CH), 125.15 (CH), 117.77 (CH), 70.24 (CH), 52.37 (CH3), 51.80 (CH2), 48.85 (CH), 32.60 (CH2), 32.53 (CH2), 26.94 (CH2), 25.33 (CH2), 24.73 (CH2), 24.66 (CH2); MS (qTOF) m/z (%) 494 (M+ + 1, 26), 423 (10), 305 (100); HRMS (qTOF) Calcd for C26H28N3O7: 494.1927. Found: 494.1915.
Methyl N-(2-(cyclohexylamino)-2-oxo-1-(p-tolyl)ethyl)-N-(3-nitro-2-oxo-2H-chromen-4-yl)glycinate (17m). Obtained from aldehyde 12c, amine 13e, isocyanide 14a and enol 15, as a pale yellow solid (122 mg, 48%); m.p. 114–116 °C; IR (cm−1) 3383, 2930, 2854, 1728, 1680, 1604, 1551, 1451, 1209, 1119, 1057, 759; 1H-NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 6,99, 1.5 Hz, 1H), 7.64 (dt, J = 7.0, 1.4 Hz, 1H), 7.40–7.33 (m, 3H), 7.26–7.17 (m, 3H), 5.81 (d, J = 8.1 Hz, 1H), 5.70 (s, 1H), 4.23 (d, J = 18.3 Hz, 1H), 3.82–3.71 (m, 1H), 3,74 (d, J = 18.3 Hz, 1H), 3.66 (s, 3H), 2.32 (s, 3H), 1.95–1.54 (m, 5H), 1.38–0.95 (m, 5H); 13C-NMR (126 MHz, CDCl3) δ 169.15 (C), 168.14 (C), 155.19 (C), 152.67 (C), 152.61 (C), 137.90 (C), 134.06 (CH), 132.85 (C), 131.83 (CH), 129.58 (CH), 128.33 (CH), 127.79 (CH), 126.85 (CH), 125.21 (CH), 118.07 (CH), 117.30 (C), 66.50 (CH), 52.62 (CH3), 51.42 (CH2), 49.00 (CH), 32.78 (CH2), 32.69 (CH2), 25.43 (CH2), 24.87 (CH2), 24.81 (CH2), 19.81 (CH3); MS (qTOF) m/z (%) 508 (M+ + 1, 67), 319 (100); HRMS (qTOF) Calcd for C27H30N3O7: 508.2084. Found: 508.2071.
Methyl N-(2-(cyclohexylamino)-2-oxo-1-(4-(trifluoromethyl)phenyl)ethyl)-N-(3-nitro-2-oxo-2H-chromen-4-yl)glycinate (17n). Obtained from aldehyde 12d, amine 13e, isocyanide 14a and enol 15, as a pale yellow solid (146 mg, 52%); m.p. 113–115 °C; IR (cm−1) 3368, 2933, 2855, 1734, 1605, 1554, 1325, 1169, 1127, 1069, 760; 1H-NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 8.1, 1.3 Hz, 1H), 7.68–7.62 (m, 3H), 7.57 (d, J = 8.2 Hz, 2H), 7.43–7.37 (m, 2H), 5.98 (d, J = 8.1 Hz, 1H), 5.42 (s, 1H), 4.14 (d, J = 17.9 Hz, 1H), 3.80 (d, J = 17.9 Hz, 1H), 3.73–3.63 (m, 1H), 3.66 (s, 3H), 1.90 –0.92 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 169.08, 166.89, 154.89, 152.76, 152.24, 138.60, 134.35, 132.66, 131.95, 131.69, 129.39, 127.70, 126.26, 125.47, 124.88, 122.71, 118.05, 117.39, 69.62, 52.62, 52.09, 49.17, 32.68, 32.63, 25.41, 24.83, 24.77; MS (qTOF) m/z (%) 568 (M+ + 1, 100), 373 (25); HRMS (qTOF) Calcd for C27H27F3N3O7: 562.1801. Found: 562.1793.
Methyl N-(2-(tert-butylamino)-2-oxo-1-phenylethyl)-N-(3-nitro-2-oxo-2H-chromen-4-yl)glycinate (17o). Obtained from aldehyde 12a, amine 13e, isocyanide 14b and enol 15, as a yellow solid (140 mg, 60%); m.p. 139–141 °C; IR (cm−1) 3378, 2969, 1685, 1605, 1554, 1456, 1365, 1213, 759; 1H-NMR (500 MHz, CDCl3) δ 8.10 (dd, J = 8.1, 1.2 Hz, 1H), 7.62 (t, J = 7.0 Hz, 1H), 7.44–7.29 (m, 7H), 5.84 (bs, 1H), 5.31 (s, 1H), 4.16 (d, J = 18.1 Hz, 1H), 3.78 (d, J = 18.1 Hz, 1H), 3.65 (s, 3H), 1.25 (s, 9H); 13C-NMR (126 MHz, CDCl3) δ 169.19 (C), 167.67 (C), 155.18 (C), 152.70 (C), 152.65 (C), 134.63 (C), 134.04 (CH), 129.63 (CH), 129.36 (CH), 129.04 (C), 128.96 (CH), 128.70 (C), 128.00 (CH), 125.24 (CH), 117.90 (CH), 70.68 (CH), 52.51 (CH3), 52.21 (C), 51.89 (CH2), 28.78 (CH3), 28.72 (CH3), 28.47 (CH3); MS (qTOF) m/z (%) 468 (M+ + 1, 15), 279 (100); HRMS (qTOF) Calcd for C24H26N3O7: 468.1771. Found: 468.1751.
Methyl N-(3-nitro-2-oxo-2H-chromen-4-yl)-N-(2-oxo-2-(pentylamino)-1-phenylethyl) glycinate (17p). Obtained from aldehyde 12a, amine 13e, isocyanide 14c and enol 15, as a yellow solid (92 mg, 38%); m.p. 72–74 °C; IR (cm−1) 3333, 2956, 1740, 1736, 1650, 1606, 1556, 1454, 1374, 1280, 1209, 1060, 761; 1H-NMR (500 MHz, CDCl3) δ 8.11 (dd, J = 8.1, 1.3 Hz, 1H), 7.63 (dt, J = 6.2, 1.4 Hz, 1H), 7.42–7.35 (m, 7H), 5.95 (t, J = 5.5 Hz, 1H), 5.40 (s, 1H), 4.16 (d, J = 17.9 Hz, 1H), 3.81 (d, J = 18.0 Hz, 1H), 3.65 (s, 3H), 3.25–3.17 (m, 2H), 1.45–1.36 (m, 2H), 1.28–1.20 (m, 2H), 1.20–1.11 (m, 2H), 0.83 (t, J = 7.2 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ 169.19 (C), 168.56 (C), 155.14 (C), 152.76 (C), 152.41 (C), 134.45 (C), 134.05 (CH), 129.75 (CH), 129.39 (CH), 129.06 (CH), 127.88 (CH), 125.34 (CH), 117.93 (CH), 117.60 (C), 70.23 (CH), 52.50 (CH3), 51.95 (CH2), 40.04 (CH2), 28.99 (CH2), 22.34 (CH2), 14.03 (CH3); MS (qTOF) m/z (%) 482 (M+ + 1, 100), 395 (37); HRMS (qTOF) Calcd for C25H28N3O7: 482.1927. Found: 482.1916.
Methyl N-(2-(benzylamino)-2-oxo-1-phenylethyl)-N-(3-nitro-2-oxo-2H-chromen-4-yl)glycinate (17q). Obtained from aldehyde 12a, amine 13e, isocyanide 14d and enol 15, as a yellow solid (118 mg, 47%); m.p. 145–147 °C; IR (cm−1) 3296, 2946, 1744, 1722, 1651, 1602, 1556, 1532, 1454, 1410, 1215, 1179, 1054, 759, 698; 1H-NMR (500 MHz, CDCl3) δ 8.10 (d, J = 7.2 Hz, 1H), 7.62 (dt, J = 7.1, 1.4 Hz, 1H), 7.42–7.36 (m, 5H), 7.34 (d, J = 7.8 Hz, 2H), 7.27–7.23 (m, 3H), 7.08 (dd, J = 6.4, 3.0 Hz, 2H), 6.32 (t, J = 5.6 Hz, 1H), 5.47 (s, 1H), 4.38 (dd, J = 5.8, 1.3 Hz, 2H), 4.14 (d, J = 18.0 Hz, 1H), 3.81 (d, J = 18.0 Hz, 1H), 3.62 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 1169.19 (C), 168.52 (C), 155.05 (C), 152.68 (C), 152.37 (C), 137.43 (C), 134.23 (C), 134.05 (CH), 129.82 (CH), 129.44 (CH), 129.07 (CH), 128.87 (CH), 127.81 (CH), 125.35 (CH), 117.93 (CH), 117.52 (C), 70.09 (CH), 52.49 (CH3), 51.93 (CH2), 43.97 (CH2); MS (qTOF) m/z (%) 502 (M+ + 1, 100), 415 (24), 299 (17); HRMS (qTOF) Calcd for C27H24N3O7: 502.1614. Found: 502.1602.
Methyl N-(2-(benzylamino)-2-oxo-1-(p-tolyl)ethyl)-N-(3-nitro-2-oxo-2H-chromen-4-yl)glycinate (17r). Obtained from aldehyde 12c, amine 13e, isocyanide 14d and enol 15, as a yellow solid (124 mg, 48%); m.p. 146–148 °C; IR (cm−1) 3294, 1747, 1720, 1655, 1601, 1554, 1530, 1452, 1436, 1368, 1209, 1178, 1053, 794; 1H-NMR (500 MHz, CDCl3) δ 8.05 (d, J = 7.4 Hz, 1H), 7.62 (t, J = 7.3 Hz, 1H), 7.38–7.20 (m, 10H), 7.14–7.11 (m, 2H), 6.26 (t, J = 5.6 Hz, 1H), 5.78 (s, 1H), 4.41 (d, J = 5.9 Hz, 2H), 4.23 (d, J = 18.3 Hz, 1H), 3.74 (d, J = 18.3 Hz, 1H), 3.64 (s, 3H), 2.29 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 169.11 (C), 169.04 (C), 155.06 (C), 152.66 (C), 152.49 (C), 137.93 (C), 137.51 (C), 134.03 (C), 132.57 (C), 131.89 (CH), 129.70 (CH), 128.92 (CH), 128.48 (CH), 127.87 (CH), 127.66 (CH), 126.9 (CH)3, 125.30 (CH), 118.09 (CH), 117.25 (C), 66.23 (CH), 52.58 (CH3), 51.45 (CH2), 44.00 (CH2), 19.77 (CH3); MS (qTOF) m/z (%) 516 (M+ + 1, 17), 429 (100), 299 (10); HRMS (qTOF) Calcd for C28H26N3O7: 516.1771. Found: 516.1782.
Ethyl 3-((2-(cyclohexylamino)-2-oxo-1-phenylethyl)(3-nitro-2-oxo-2H-chromen-4-yl)amino)propanoate (17s). Obtained from aldehyde 12a, amine 13f, isocyanide 14a and enol 15, as a yellow solid (131 mg, 50%); m.p. 130–131 °C; IR (cm−1) 3367, 2931, 2854, 1732, 1682, 1603, 1553, 1451, 1200, 1052, 762; 1H-NMR (500 MHz, CDCl3) δ 8.05 (dd, J = 8.1, 1.2 Hz, 1H), 7.65 (d, J = 7,3, 1.3 Hz, 1H), 7.45–7.34 (m, 7H), 5.99 (d, J = 8.0 Hz, 1H), 5.02 (s, 1H), 4.01 (q, J = 7.1 Hz, 2H), 3.83 (dt, J = 14.6, 7.2 Hz, 1H), 3.70–3.62 (m, 1H), 3.43–3.36 (m, 1H), 2.62 (dt, J = 17.0, 7.1 Hz, 1H), 2.44 (dt, J = 17.1, 6.2 Hz, 1H), 1.91 (d, J = 10.9 Hz, 1H), 1.75–1.52 (m, 5H), 1.36–1.16 (m, 3H), 1.13 (t, J = 9.0 Hz, 3H), 0.98 (ddd, J = 23.4, 12.1, 3.4 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 171.43 (C), 167.65 (C), 155.05 (C), 153.46 (C), 152.85 (C), 134.86 (C), 134.03 (CH), 132.08 (C), 129.61 (CH), 129.32 (CH), 128.97 (CH), 127.89 (CH), 125.25 (CH), 118.04 (C), 117.89 (CH), 70.02 (CH), 61.03 (CH2), 49.14 (CH), 47.30 (CH2), 32.63 (CH2), 32.54 (CH2), 32.52 (CH2), 27.05 (CH2), 25.49 (CH2), 24.94 (CH2), 24.83 (CH2), 14.12 (CH3); MS (qTOF) m/z (%) 522 (M+ + 1, 100), 481 (17), 333 (20); HRMS (qTOF) Calcd for C28H32N3O7: 522.2240. Found: 522.2231.
3.4.3. General Procedure for the Reduction of Nitro Derivatives 17a–s
To a vigorously stirred solution of enol-Ugi adduct
17a–
s (0.4 mmol) in glacial acetic acid (8 mL), iron powder (9.6 mmol, 24 equiv) was added in one portion. The reaction mixture was stirred at rt for 2–4 h. Then water (50 mL) and dichloromethane (25 mL) were added. The unreacted iron was removed by filtration and the filtrate transferred to a separatory funnel. The phases were separated, and the aqueous layer extracted again with dichloromethane (25 mL). The combined organic extracts were washed with water (25 mL), saturated NaHCO
3 (10 mL) and water again (25 mL), and then dried (Na
2SO
4) and evaporated to dryness. The residue was purified by flash column chromatography (SiO
2, gradient from 100 % hexanes to hexanes–AcOEt 7:3) to give, depending on the case, chromeno[3,4-
b]piperazines
19a–
i,s, aminocoumarins
18j,
k or chromeno[3,4-
b]piperazines
20l–
r (
Table 1 and
Table 2).
Synthesis and Characterization of chromeno[3,4-b]piperazines 19a–i,s
1-Benzyl-2-phenyl-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19a). Obtained from 17a as a pale yellow solid (130 mg, 85%); m.p. 183–185 °C; IR (cm−1) 3254, 1677, 1620, 1567, 1495, 1465, 1427, 1357, 1182, 1101, 1046, 746, 701; 1H-NMR (500 MHz, CDCl3) δ 7.98 (dd, J = 8.0, 1.3 Hz, 1H), 7.84 (bs, 1H), 7.53 (dt, J = 7.3, 1.5 Hz, 1H), 7.41 (t, J = 7.3 Hz, 2H), 7.39–7.30 (m, 8H), 7.28–7.23 (m, 2H), 4.98 (s, 1H), 4.87 (d, J = 15.2 Hz, 1H), 4.71 (d, J = 15.2 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 163.37 (C), 156.63 (C), 150.85 (C), 135.88 (C), 135.67 (C), 130.46 (CH), 129.33 (CH), 129.02 (CH), 128.75 (CH), 128.47 (CH), 127.93 (CH), 126.04 (CH), 125.15 (CH), 123.34 (CH), 118.01 (CH), 116.55 (C), 112.25 (C), 63.91 (CH), 58.03 (CH2); HRMS (qTOF) Calcd for C24H19N2O3: 383.1396. Found: 383.1381.
1-Benzyl-2-(2-bromophenyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19b). Obtained from 17b as a pale yellow solid (155 mg, 84%); m.p. 240–242 °C; IR (cm−1) 3195, 3085, 2938, 1700, 1688, 1625, 1495, 1467, 1392, 1346, 1094, 755, 699; 1H-NMR (500 MHz, CDCl3) δ 7.89 (bs, 1H), 7.74 (dd, J = 8.0, 1.1 Hz, 1H), 7.61 (dd, J = 7.7, 1.3 Hz, 1H), 7.48 (dt, J = 6.9, 1.3 Hz, 1H), 7.41–7.28 (m, 5H), 7.20–7.10 (m, 4H), 7.04 (dd, J = 7.5, 1.8 Hz, 1H), 5.33 (s, 1H), 5.03 (d, J = 14.7 Hz, 1H), 4.87 (d, J = 14.7 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 162.69 (C), 156.79 (C), 150.85 (C), 136.26 (C), 135.91 (C), 135.69 (C), 133.90 (CH), 130.47 (CH), 130.30 (CH), 129.21 (CH), 128.91 (CH), 128.33 (CH), 128.16 (CH), 128.08 (CH), 124.93 (CH), 124.53 (C), 124.12 (CH), 117.82 (CH), 116.47 (C), 111.90 (C), 64.07 (CH2), 59.43 (CH); HRMS (qTOF) Calcd for C24H18BrN2O3: 461.0501. Found: 461.0473.
1-Benzyl-2-(p-tolyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19c). Obtained from 17c as a white solid (124 mg, 78%); m.p. 170–172 °C; IR (cm−1) 3396, 3272, 1692, 1619, 1495, 1363, 1206, 1113, 755; 1H-NMR (500 MHz, CDCl3) δ 7.96 (dd, J = 8.0, 1.3 Hz, 1H), 7.79 (bs, 1H), 7.52 (dt, J = 7.4, 1.4 Hz, 1H), 7.43–7.30 (m, 7H), 7.23 (d, J = 8.1 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 4.93 (s, 1H), 4.86 (d, J = 15.2 Hz, 1H), 4.69 (d, J = 15.2 Hz, 1H), 2.26 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 163.50 (C), 156.63 (C), 150.86 (C), 138.32 (C), 135.97 (C), 135.86 (C), 132.76 (C), 130.38 (CH), 129.71 (CH), 129.32 (CH), 128.71 (CH), 127.95 (CH), 125.99 (CH), 125.09 (CH), 123.37 (CH), 117.98 (CH), 116.65 (C), 112.29 (C), 63.79 (CH), 57.99 (CH2), 21.12 (CH3); MS (qTOF) m/z (%) 397 (M+ + 1, 100), 337 (30); HRMS (qTOF) Calcd for C25H21N2O3: 397.1552. Found: 397.1544.
1-Benzyl-2-(4-(trifluoromethyl)phenyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19d). Obtained from 17d as a pale yellow solid (160 mg, 89%); m.p. 172–174 °C; IR (cm−1) 3438, 3260, 1684, 1620, 1498, 1469, 1414, 1361, 1327, 1169, 1115, 1069, 752, 732; 1H-NMR (500 MHz, CDCl3) δ 7.99 (d, J = 6.8 Hz, 1H), 7.84 (bs, 1H), 7.57–7.48 (m, 5H), 7.44 (d, J = 8.5 Hz, 2H), 7.39–7.30 (m, 5H), 5.02 (s, 1H), 4.84 (d, J = 15.1 Hz, 1H), 4.70 (d, J = 15.1 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 162.77 (C), 156.50 (C), 150.88 (C), 139.60 (C), 135.56 (C), 135.42 (C), 130.73 (CH), 129.45 (CH), 128.97 (CH), 128.00 (CH), 126.53 (CH), 126.06 (CH), 126.03 (C), 125.38 (CH), 123.11 (CH), 118.16 (CH), 116.42 (C), 112.72 (C), 63.64 (CH), 58.15 (CH2); MS (qTOF) m/z (%) 451 (M+ + 1, 95), 391 (100); HRMS (qTOF) Calcd for C25H18F3N2O3: 451.1270. Found: 451.1264.
1-(Benzo[d][1,3]dioxol-5-ylmethyl)-2-phenyl-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19e). Obtained from 17e as a pale yellow solid (147 mg, 86%); m.p. 174–176 °C; IR (cm−1) 3264, 1692, 1626, 1500, 1445, 1375, 1354, 1319, 1253, 1180, 1106, 1039, 926, 851, 747, 704; 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 4.0 Hz, 1H), 7.95 (d, J = 1.2 Hz, 1H), 7.53 (dt, J = 7.0 Hz, 1.4 Hz, 1H), 7.41 (d, J = 7.8 Hz, 2H), 7.36 (dd, J = 7.0, 1.5 Hz, 2H), 7.28–7.22 (m, 3H), 6.80–6.75 (m, 3H), 5.96 (s, 2H), 5.00 (s, 1H), 4.79 (d, J = 14.9 Hz, 1H), 4.59 (d, J = 14.9 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 163.44 (C), 156.59 (C), 150.80 (C), 148.52 (C), 148.02 (C), 135.73 (C), 135.65 (C), 130.41 (CH), 129.55 (C), 128.99 (CH), 128.42 (CH), 125.99 (CH), 125.09 (CH), 123.32 (CH), 121.76 (CH), 117.98 (CH), 116.48 (C), 112.21 (C), 108.78 (CH), 108.06 (CH), 101.46 (CH2), 63.46 (CH), 57.69 (CH2); MS (qTOF) m/z (%) 427 (M+ + 1, 100), 427 (15), 274 (18); HRMS (qTOF) Calcd for C25H19N2O5: 427.1294. Found: 427.1293.
1-(Benzo[d][1,3]dioxol-5-ylmethyl)-2-(p-tolyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19f). Obtained from 17f as a pale yellow solid (151 mg, 86%); m.p. 213–215 °C; IR (cm−1) 3408, 3192, 1715, 1677, 1625, 1502, 1419, 1326, 1298, 1109, 1036, 805, 751; 1H-NMR (500 MHz, CDCl3) δ 7.93 (dd, J = 8.0, 1.2 Hz, 1H), 7.76 (bs, 1H), 7.52 (dt, J = 7.3, 1.5 Hz, 1H), 7.43–7.36 (m, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 6.77 (d, J = 0.8 Hz, 2H), 6.76 (s, 1H), 5.96 (d, J = 0.9 Hz, 2H), 4.94 (s, 1H), 4.77 (d, J = 14.9 Hz, 1H), 4.57 (d, J = 14.9 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 163.52 (C), 156.64 (C), 150.85 (C), 148.57 (C), 148.06 (C), 138.34 (C), 135.77 (C), 132.68 (C), 130.42 (CH), 129.73 (CH), 129.64 (C), 125.94 (CH), 125.09 (CH), 123.38 (CH), 121.78 (CH), 118.02 (CH), 116.57 (C), 112.16 (C), 108.82 (CH), 108.08 (CH), 101.50 (CH2), 63.35 (CH), 57.69 (CH2), 21.14 (CH3); MS (qTOF) m/z (%) 441 (M+ + 1, 100), 400 (20), 281(30); HRMS (qTOF) Calcd for C26H21N2O5: 441.4630. Found: 441.1449.
1-(Benzo[d][1,3]dioxol-5-ylmethyl)-2-(4-(trifluoromethyl)phenyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19g). Obtained from 17g as a pale yellow solid (148 mg, 75%); m.p. 190-192 °C; IR (cm−1) 3080, 2918, 1685, 1619, 1493, 1412, 1329, 1241, 1114, 1068, 1041, 998, 756; 1H-NMR (500 MHz, CDCl3) δ 7.96 (dd, J = 6.8, 1.6 Hz, 1H), 7.81 (bs, 2H), 7.58–7.48 (m, 5H), 7.46–7.41 (m, 2H), 6.80–6.75 (m, 3H), 5.97 (dd, J = 2.5, 1.3 Hz, 2H), 5.03 (s, 1H), 4.75 (d, J = 14.8 Hz, 1H), 4.58 (d, J = 14.8 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 162.80 (C), 156.49 (C), 150.87 (C), 148.71 (C), 148.29 (C), 139.61 (C), 135.30 (C), 130.72 (CH), 129.22 (C), 126.51 (CH), 126.07 (CH), 126.04 (CH), 125.35 (CH), 123.11 (CH), 121.91 (CH), 118.18 (CH), 116.38 (C), 112.69 (C), 108.92 (CH), 108.10 (CH), 101.59 (CH2), 63.28 (CH), 57.89 (CH2); MS (qTOF) m/z (%) 495 (M+ + 1, 100), 339 (30), 353 (65); HRMS (qTOF) Calcd for C26H18F3N2O5: 495.1168. Found: 495.1157.
1-Cyclohexyl-2-(p-tolyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19h). Obtained from 17h as a pale yellow solid (107 mg, 69%); m.p. 249–251 °C; IR (cm−1) 3444, 2931, 2853, 1692, 1622, 1495, 1406, 1335, 1102, 999, 757; 1H-NMR (500 MHz, CDCl3) δ 7.90 (bs, 1H), 7.74 (dd, J = 7.9, 1.4 Hz, 1H), 7.49 (dt, J = 6.0, 1.5 Hz, 1H), 7.42–7.36 (m, 2H), 7.28 (d, J = 8.0 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 5.17 (s, 1H), 3.77 (tt, J = 12.0, 3.7 Hz, 1H), 2.31 (d, J = 13.3 Hz, 1H), 2.25 (s, 3H), 1.95 (d, J = 13.2 Hz, 1H), 1.83–1.09 (m, 8H); 13C-NMR (126 MHz, CDCl3) δ 164.43 (C), 156.66 (C), 150.87 (C), 138.08 (C), 136.15 (CH), 133.12 (C), 130.15 (C), 129.60 (CH), 125.84 (CH), 124.98 (CH), 123.36 (CH), 117.92 (CH), 117.05 (C), 112.33 (C), 62.44 (CH), 58.98 (CH), 31.92 (CH3), 31.76 (CH3), 26.07 (CH3), 25.98 (CH3), 25.24 (CH3), 21.10 (CH3); MS (qTOF) m/z (%) 389 (M+ + 1, 100), 348 (31), 255 (29); HRMS (qTOF) Calcd for C24H25N2O3: 389.1865. Found: 389.1866.
1-Cyclohexyl-2-(4-(trifluoromethyl)phenyl)-1,4-dihydro-2H-chromeno[3,4-b]pyrazine-3,5-dione (19i). Obtained from 17i as a pale yellow solid (127 mg, 72%); m.p. 113–115 °C; IR (cm−1) 3427, 2932, 2856, 1692, 1622, 1411, 1326, 1165, 1125, 1069, 755; 1H-NMR (500 MHz, CDCl3) δ 8.00 (bs, 1H), 7.75 (dd, J = 7.9, 1.2 Hz, 1H), 7.57–7.50 (m, 6H), 7.45–7.36 (m, 2H), 5.24 (s, 1H), 3.79 (tt, J = 12.0, 3.6 Hz, 1H), 2.30 (d, J = 12.6 Hz, 1H), 1.96 (d, J = 13.1 Hz, 1H), 1.85–1.58 (m, 5H), 1.29–1.11 (m, 3H); 13C-NMR (126 MHz, CDCl3) δ 163.73 (C), 156.52 (C), 150.91 (C), 140.21 (C), 135.73 (C), 130.46 (CH), 126.42 (CH), 125.96 (CH), 125.93 (CH), 125.20 (CH), 123.11 (CH), 118.10 (CH), 116.80 (C), 112.54 (C), 62.62 (CH), 59.06 (CH), 31.86 (CH2), 31.73 (CH2), 27.07 (CH2), 25.95 (CH2), 25.21 (CH2); MS (qTOF) m/z (%) 443 (M+ + 1, 100), 301 (<5); HRMS (qTOF) Calcd for C24H22F3N2O3: 443.1583. Found: 443.1578.
Ethyl 3-(3,5-dioxo-2-phenyl-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)propanoate (19s). Obtained from 17s as a white solid (133 mg, 85%); m.p. 145–147 °C; IR (cm−1) 3231, 1733, 1680, 1619, 1498, 1460, 1372, 1188, 1126, 1021, 757; 1H-NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.89 (dd, J = 7.9, 1.1 Hz, 1H), 7.52 (dt, J = 7.3, 1.2 Hz, 1H), 7.43–7.36 (m, 4H), 7.29–7.24 (m, 3H), 5.09 (s, 1H), 4.17–4.06 (m, 3H), 3.89 (ddd, J = 14.7, 8.2, 6.4 Hz, 1H), 2.79 (ddd, J = 14.6, 8.1, 6.4 Hz, 1H), 2.73–2.63 (m, 1H), 1.19 (t, J = 7.1 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ 170.71 (C), 163.61 (C), 156.53 (C), 150.72 (C), 135.51 (C), 135.15 (C), 130.45 (CH), 129.06 (CH), 128.57 (CH), 125.81 (CH), 125.15 (CH), 123.39 (CH), 117.98 (CH), 116.43 (C), 112.23 (C), 64.73 (CH), 61.31 (CH2), 50.18 (CH2), 33.96 (CH2), 14.15 (CH3); HRMS (qTOF) Calcd for C22H21N2O5: 393.1450. Found: 393.1444.
Synthesis and Characterization of Aminocoumarins 18j,k
2-((3-Amino-2-oxo-2H-chromen-4-yl)(phenyl)amino)-N-cyclohexyl-2-phenylacetamide (18j). Obtained from 17j as a white solid (99 mg, 53%); m.p. 203–205 °C; IR (cm−1) 3466, 2933, 2852, 1719, 1635, 1600, 1556, 1497, 1455, 1177, 746; 1H-NMR (500 MHz, CDCl3) δ 7.27–7.18 (m, 4H), 7.11–7.05 (m, 3H), 7.04–6.96 (m, 4H), 6.83 (t, J = 7.3 Hz, 1H), 6.64 (d, J = 8.1 Hz, 2H), 6.21 (bs, 2H), 5.65 (d, J = 8.1 Hz, 1H), 5.51 (s, 1H), 3.93–3.83 (m, 1H), 2.15–0.92 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 171.49 (C), 160.18 (C), 147.29 (C), 145.30 (C), 134.85 (C), 132.81 (C), 129.70 (CH), 129.48 (CH), 129.42 (CH), 128.12 (CH), 125.71 (CH), 123.94 (CH), 122.65 (CH), 121.88 (C), 119.66 (C), 118.93 (CH), 115.93 (CH), 112.72 (CH), 68.52 (CH), 49.44 (CH), 33.19 (CH2), 32.69 (CH2), 25.55 (CH2), 24.98 (CH2), 24.84 (CH2); MS (qTOF) m/z (%) 468 (M+ + 1, 5, 407 (18), 369 (100); HRMS (qTOF) Calcd for C29H30N3O3: 468.2287. Found: 468.2285.
2-((3-Amino-2-oxo-2H-chromen-4-yl)(phenyl)amino)-N-cyclohexyl-2-(3,4-dimethoxyphenyl)acetamide (18k). Obtained from 17k as a white solid (163 mg, 77%); m.p. 243–245 °C; IR (cm−1) 3439, 3326, 2938, 2850, 1715, 1637, 1599, 1539, 1518, 1464, 1253, 1176, 1152, 1028, 763, 749; 1H-NMR (500 MHz, CDCl3) δ 7.20 (t, J = 7.9 Hz, 2H), 7.11–7.06 (m, 2H), 6.98 (d, J = 3.5 Hz, 2H), 6.82–6.78 (m, 2H), 6.74 (s, 1H), 6.61 (d, J = 8.2 Hz, 2H), 6.50 (d, J = 8.3 Hz, 1H), 6.20 (bs, 2H), 5.60 (d, J = 8.1 Hz, 1H), 5.44 (s, 1H), 3.90–3.80 (m, 1H), 3.71 (s, 3H), 3.52 (s, 3H), 2.08–0.79 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 171.68 (C), 160.15 (C), 149.69 (C), 148.24 (C), 147.43 (C), 145.24 (C), 134.75 (C), 129.71 (CH), 125.75 (CH), 124.96 (CH), 123.95 (CH), 122.57 (CH), 121.99 (C), 119.86 (CH), 118.89 (CH), 116.26 (CH), 112.65 (CH), 110.32 (CH), 68.09 (CH), 55.87 (CH3), 55.79 (CH3), 49.43 (CH), 33.21 (CH2), 32.77 (CH2), 29.84 (CH2), 24.98 (CH2), 24.86 (CH2); MS (qTOF) m/z (%) 528 (M+ + 1, 100), 369 (25), 276 (13); HRMS (qTOF) Calcd for C31H34N3O5: 528.2498. Found: 528.2490.
Synthesis and Characterization of chromeno[3,4-b]piperazines 20l-r
N-cyclohexyl-2-(3,5-dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-2-phenyl acetamide (20l). Obtained from 17l as a pale yellow solid (121 mg, 70%); m.p. 240–242 °C; IR (cm−1) 3268, 2931, 2854, 1703, 1818, 1544, 1496, 1451, 1382, 1111, 752; 1H-NMR (500 MHz, CDCl3) δ 7.63 (dd, J = 8.0, 1.2 Hz, 1H), 7.54 (bs, 1H), 7.51 (dt, J = 7.1, 1.4 Hz, 1H), 7.43–7.39 (m, 4H), 7.34–7.29 (m, 3H), 5.79 (d, J = 8.0 Hz, 1H), 5.37 (s, 1H), 3.98–3.90 (m, 1H), 3.92 (s, 3H), 2.03–1.90 (m, 2H), 1.74–1.57 (m, 4H), 1.45–1.07 (m, 4H.; 13C-NMR (126 MHz, CDCl3) δ 167.85 (C), 163.00 (C), 156.92 (C), 150.63 (C), 135.72 (C), 135.01 (C), 130.32 (CH), 129.52 (CH), 128.50 (CH), 125.06 (CH), 122.96 (CH), 118.02 (CH), 116.33 (C), 114.08 (C), 69.14 (CH), 49.49 (CH2), 49.05 (CH), 33.17 (CH2), 33.10 (CH2), 25.51 (CH2), 24.89 (CH2), 24.85 (CH2); MS (qTOF) m/z (%) 432 (M+ + 1, 100), 305 (<5), 234 (<5); HRMS (qTOF) Calcd for C25H26N3O4: 432.1923. Found: 432.1914.
N-cyclohexyl-2-(3,5-dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-2-(p-tolyl)acetamide (20m). Obtained from 17m as a pale yellow solid (98 mg, 55%); m.p. 245–247 °C (dec.); IR (cm−1) 3400, 3280, 2930, 2849, 1701, 1651, 1618, 1561, 1439, 1412, 1347, 1108, 751, 732; 1H-NMR (500 MHz, CDCl3) δ 7.67 (bs, 1H), 7.47 (t, J = 7.2 Hz, 1H), 7.44–7.37 (m, 3H), 7.35–7.31 (m, 2H), 7.26–7.19 (m, 2H), 5.72 (d, J = 8.1 Hz, 1H), 5.59 (s, 1H), 4.09 (d, J = 16.7 Hz, 1H), 3.98 (d, J = 16.7 Hz, 1H), 3.95–3.87 (m, 1H), 1.98–0.95 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 168.26 (C), 162.61 (C), 156.91 (C), 150.61 (C), 137.43 (C), 136.82 (C), 133.84 (C), 131.87 (CH), 130.36 (CH), 129.58 (CH), 128.28 (CH), 127.23 (CH), 124.88 (CH), 123.08 (CH), 118.05 (CH), 116.39 (C), 111.84 (C), 67.33 (CH), 49.97 (CH), 49.08 (CH2), 33.00 (CH2), 25.47 (CH2), 24.81 (CH2), 19.44 (CH3); MS (qTOF) m/z (%) 446 (M+ + 1, 11), 319 (100); HRMS (qTOF) Calcd for C26H28N3O4: 446.2080. Found: 446.2074.
N-cyclohexyl-2-(3,5-dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-2-(4-(trifluoromethyl)phenyl)acetamide (20n). Obtained from 17n as a pale yellow solid (162 mg, 81%); m.p. 249–251 °C (dec.); IR (cm−1) 3313, 3266, 2930, 2855, 1704, 1688, 1620, 1549, 1497, 1487, 1326, 1167, 1126, 1068, 824, 1H-NMR (500 MHz, CDCl3) δ 7.68 (d, J = 8.2 Hz, 1H), 7.60 (bs, 1H), 7.58 (dd, J = 8.1, 1.2 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.45–7.34 (m, 3H), 7.39–7.34 (m, 1H), 5.88 (d, J = 4.7 Hz, 1H), 5.37 (s, 1H), 3.98–3.88 (m, 1H), 3.91 (d, J = 2.9 Hz, 2H), 2.04–1.09 (m, 10H); 13C-NMR (126 MHz, CDCl3) δ 167.11 (C), 162.84 (C), 156.76 (C), 150.56 (C), 138.72 (C), 134.97 (C), 130.56 (CH), 128.98 (CH), 127.12 (C), 126.43 (CH), 125.26 (CH), 122.53 (CH), 118.18 (CH), 116.04 (C), 114.70 (C), 68.39 (CH), 49.41 (CH2), 49.20 (CH), 33.18 (CH2), 33.11 (CH2), 25.44 (CH2), 24.88 (CH2); MS (qTOF) m/z (%) 522 (M+ + Na+, 100), 500 (M+ + 1, 54), 429 (10); HRMS (qTOF) Calcd for C26H25F3N3O4: 500.1797. Found: 500.1782.
N-(tert-butyl)-2-(3,5-dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-2-phenylacetamide (20o). Obtained from 17o as a pale yellow solid (94 mg, 58%); m.p. 258–260 °C (dec.); IR (cm−1) 3328, 3269, 2966, 2931, 1680, 1619, 1561, 1496, 1466, 1365, 1288, 1111, 750; 1H-NMR (500 MHz, CDCl3) δ .59 (dd, J = 8.0, 1.2 Hz, 1H), 7.53–7.49 (m, 2H), 7.44–7.40 (m, 4H), 7.34–7.29 (m, 3H), 5.71 (bs, 1H), 5.31 (s, 1H), 3.96 (q, J = 16.9 Hz, 2H), 1.41 (s, 9H); 13C-NMR (126 MHz, CDCl3) δ 168.11 (C), 163.00 (C), 156.94 (C), 150.63 (C), 135.98 (C), 135.19 (C), 130.34 (CH), 129.54 (CH), 129.50 (CH), 128.40 (CH), 125.04 (CH), 123.00 (CH), 118.01 (CH), 116.32 (C), 113.75 (C), 69.53 (CH), 52.50 (C), 49.54 (CH2), 28.85 (CH3); MS (qTOF) m/z (%) 406 (M+ + 1, 60), 321 (27), 279 (100); HRMS (qTOF) Calcd for C23H24N3O4: 406.1767. Found: 406.1761.
2-(3,5-Dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-N-pentyl-2-phenylacetamide (20p). Obtained from 17p as a white solid (92 mg, 55%); m.p. 206–208 °C (dec.); IR (cm−1) 3310, 3276, 2929, 1687, 1616, 1567, 1496, 1471, 1380, 1115, 999, 931, 748, 726; 1H-NMR (500 MHz, CDCl3) δ 7.63 (dd, J = 8.0, 1.2 Hz, 1H), 7.51 (dt, 6.9, J = 1.3 Hz, 2H), 7.43–7.37 (m, 4H), 7.35–7.28 (m, 4H), 6.04 (t, J = 5.4 Hz, 1H), 5.39 (s, 1H), 3.92 (d, J = 1.7 Hz, 2H), 3.37 (dd, J = 13.2, 7.0 Hz, 2H), 1.57–1.50 (m, 2H), 1.37–1.25 (m, 4H), 0.90 (t, J = 7.1 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ 168.65 (C), 162.80 (C), 156.78 (C), 150.47 (C), 135.51 (C), 134.77 (C), 130.21 (CH), 129.44 (CH), 129.39 (CH), 128.38 (CH), 124.94 (CH), 122.83 (CH), 117.87 (CH), 116.16 (C), 114.07 (C), 69.04 (CH), 49.40 (CH2), 40.00 (CH2), 29.19 (CH2), 29.04 (CH2), 22.27 (CH2), 13.97 (CH3); MS (qTOF) m/z (%) 420 (M+ + 1, 14), 293 (100); HRMS (qTOF) Calcd for C24H26N3O4: 420.1923. Found: 420.1918.
N-benzyl-2-(3,5-dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-2-phenylacetamide (20q). Obtained from 17q as a white solid (118 mg, 67%); m.p. 238–240 °C (dec.); IR (cm−1) 3280, 1686, 1618, 1496, 1379, 1113, 751, 700; 1H-NMR (500 MHz, CDCl3) δ 7.59 (dd, J = 8.0, 1.1 Hz, 1H), 7.52–7.44 (m, 2H), 7.42–7.33 (m, 6H), 7.33–7.25 (m, 6H), 6.33 (t, J = 5.5 Hz, 1H), 5.42 (s, 1H), 4.56 (qd, J = 14.6, 5.8 Hz, 2H), 3.92 (d, J = 1.7 Hz, 2H); 13C-NMR (126 MHz, CDCl3) δ 168.90 (C), 162.97 (C), 156.87 (C), 150.57 (C), 137.66 (C), 135.55 (C), 134.61 (C), 130.39 (CH), 129.63 (CH), 129.52 (CH), 129.06 (CH), 128.55 (CH), 128.06 (CH), 128.01 (CH), 125.10 (CH), 122.89 (CH), 118.00 (CH), 116.18 (C), 114.29 (C), 69.11 (CH), 49.43 (CH2), 44.15 (CH2); MS (qTOF) m/z (%) 440 (M+ + 1, 100), 282 (40), 169 (15); HRMS (qTOF) Calcd for C26H22N3O4: 440.1610. Found: 440.1585.
N-benzyl-2-(3,5-dioxo-3,4-dihydro-2H-chromeno[3,4-b]pyrazin-1(5H)-yl)-2-(p-tolyl)acetamide (20r). Obtained from 17r as a white solid (141 mg, 78%); m.p. 221–223 °C (dec.); IR (cm−1) 3369, 1711, 1665, 1649, 1559, 1495, 1113, 754, 703; 1H-NMR (500 MHz, CDCl3) δ 7.79 (bs, 1H), 7.58 (dt, J = 7.2, 1.2 Hz, 1H), 7.54 (dd, J = 8.1, 1.2 Hz, 1H), 7.51–7.54 (m, 5H), 7.42–7.38 (m, 5H), 7.36–7.23 (m, 2H), 6.38 (t, J = 6.4 Hz, 1H), 5.79 (s, 1H), 4.75 (dd, J = 14.6, 6.2 Hz, 1H), 4.57 (dd, J = 14.6, 5.5 Hz, 1H), 4.21 (d, J = 16.7 Hz, 1H), 4.09 (d, J = 16.7 Hz, 1H), 2.14 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 169.46 (C), 162.69 (C), 156.85 (C), 150.57 (C), 137.65 (C), 137.27 (C), 136.89 (C), 133.46 (C), 131.91 (CH), 130.43 (CH), 129.69 (CH), 129.06 (CH), 128.65 (CH), 128.12 (CH), 128.06 (CH), 127.18 (CH), 124.95 (CH), 123.05 (CH), 118.07 (CH), 116.26 (C), 112.10 (C), 66.93 (CH), 49.79 (CH2), 44.24 (CH2), 19.44 (CH3); MS (qTOF) m/z (%) 454 (M+ + 1, 27), 369 (73), 327 (100); HRMS (qTOF) Calcd for C27H24N3O4: 454.1767. Found: 454.1761.