Hydrodemethoxylation/Dealkylation on Bifunctional Nanosized Zeolite Beta
Abstract
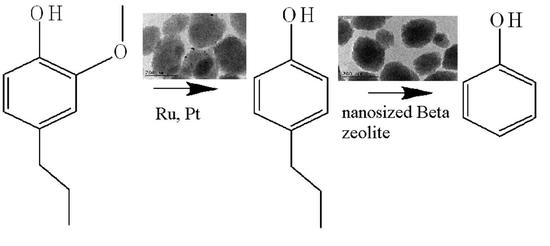
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Properties
2.2. Catalytic Activity for Phenol Preparation
3. Experimental
3.1. Materials
3.2. Synthesis of Nanosized Beta Zeolite
3.3. Impregnation with Ni, Ru, or/and Pt Nanoparticles of Nanosized Beta Zeolite
3.4. Characterization
3.5. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, K.; Fasahati, P.; Maravelias, C.T. System-Level Analysis of Lignin Valorization in Lignocellulosic Biorefineries. iScience 2020, 23, 100751. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lombardo, L.; Gözaydın, G.; Dyson, P.J.; Yan, N. Single-step conversion of lignin monomers to phenol: Bridging the gap between lignin and high-value chemicals Chinese. J. Catal. 2018, 39, 1445–1452. [Google Scholar] [CrossRef]
- Romero, Y.; Richard, F.; Brunet, S. Hydrodeoxygenation of 2-ethylphenol as a model compound of bio-crude over sulfided Mo-based catalysts: Promoting effect and reaction mechanism. Appl. Catal. B Environ. 2010, 98, 213–223. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Catalytic depolymerization of alkali lignin in subcritical water: Influence of formic acid and Pd/C catalyst on the yields of liquid monomeric aromatic products. Green Chem. 2014, 16, 4740–4748. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerisation. Science 2016, 354, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef]
- Deuss, P.J.; Scott, M.; Tran, F.; Westwood, N.J.; de Vries, J.G.; Barta, K. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C-O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. Int. Ed. 2015, 54, 258–262. [Google Scholar] [CrossRef]
- Liao, Y.; d’Halluin, M.; Makshina, E.; Verboekend, D.; Sels, B.F. Shape selectivity vapor-phase conversion of lignin-derived 4-ethylphenol to phenol and ethylene over acidic aluminosilicates: Impact of acid properties and pore constraint. Appl. Catal. B Environ. 2018, 234, 117–129. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Umezawa, T.; Nakasaka, Y.; Masuda, T. Conversion of alkylphenol to phenol via transalkylation using zeolite catalysts. Catal. Today 2020, 347, 110–114. [Google Scholar] [CrossRef]
- Verboekend, D.; Liao, Y.; Schutyser, W.; Sels, B.F. Alkylphenols to phenol and olefins by zeolite catalysis: A pathway to valorize raw and fossilized lignocellulose. Green Chem. 2016, 18, 297–306. [Google Scholar] [CrossRef]
- Sun, J.; Karim, A.M.; Zhang, H.; Kovarik, L.; Li, X.S.; Hensley, A.J.; McEwen, J.-S.; Wang, Y. Carbon-supported bimetallic Pd–Fe catalysts for vapor-phase hydrodeoxygenation of guaiacol. J. Catal. 2013, 306, 47–57. [Google Scholar] [CrossRef]
- Echeandia, S.; Pawelec, B.; Barrio, V.L.; Arias, P.L.; Cambra, J.F.; Loricera, C.V.; Fierro, J.L.G. Enhancement of phenol hydrodeoxygenation over Pd catalysts supported on mixed HY zeolite and Al2O3. An approach to O-removal from bio-oils. Fuel 2014, 117, 1061–1073. [Google Scholar] [CrossRef]
- Ohta, H.; Feng, B.; Kobayashi, H.; Haraa, K.; Fukuoka, A. Selective hydrodeoxygenation of lignin-related 4-propylphenol into n-propylbenzene in water by Pt-Re/ZrO2 catalysts. Catal. Today 2014, 234, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.B.; Lee, J.; Ha, J.M.; Lee, H.; Suh, D.J.; Jun, C.H.; Jae, J. Effective hydrodeoxygenation of lignin-derived phenols using bimetallic RuRe catalysts: Effect of carbon supports. Catal. Today 2018, 303, 191–199. [Google Scholar] [CrossRef]
- Roldán, R.; Beale, A.M.; Sánchez-Sánchez, M.; Romero-Salguero, F.J.; Jiménez-Sanchidrián, C.; Gómez, J.P.; Sanka, G. Effect of the impregnation order on the nature of metal particles of bi-functional Pt/Pd-supported zeolite Beta materials and on their catalytic activity for the hydroisomerization of alkanes. J. Catal. 2008, 254, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Gamliela, D.P.; Karakalos, S.; Valla, J.A. Liquid phase hydrodeoxygenation of anisole, 4-ethylphenol and benzofuran using Ni, Ru and Pd supported on USY zeolite. Appl. Catal. A Gen. 2018, 559, 20–29. [Google Scholar] [CrossRef]
- Wang, X.; Araia, M.; Wua, Q.; Zhang, C.; Zhao, F. Hydrodeoxygenation of lignin-derived phenolics—A review on the active sites of supported metal catalysts. Green Chem. 2020, 22, 8140–8168. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, W.; Guo, Z.; Ma, J.; Li, R. Mesoporous Beta Zeolite Catalysts for Benzylation of Naphthalene: Effect of Pore Structure and Acidity. Catalysts 2018, 8, 504. [Google Scholar] [CrossRef] [Green Version]
- Montouillout, V.; Aiello, S.; Fayon, F.; Fernandez, C. Characterization of acid sites in dehydrated H-Beta zeolite by solid state NMR. Stud. Surf. Sci. Catal. 2002, 142, 383–390. [Google Scholar]
- Engelhardt, G.; Lohse, U.; Lippmaa, E.; Tarmak, M.; Mägi, M.Z. 29Si NMR -Untersuchungen zur Verteilung der Silicium-und Aluminiumatome im Alumosilicatgitter von Zeolithen mit Faujasit-Struktur. Anorg. Allg. Chem. 1981, 482, 49–64. [Google Scholar] [CrossRef]
- Majano, G.; Mintova, S.; Ovsitser, O.; Mihailova, B.; Bein, T. Zeolite Beta nanosized assemblies. Micropor. Mesopor. Mater. 2005, 80, 227–235. [Google Scholar] [CrossRef]
- Kuehl, G.H.; Kyung, H.; Timken, C. Acid sites in zeolite Beta: Effects of ammonium exchange and steaming. Micropor. Mesopor. Mater. 2000, 35–36, 521–532. [Google Scholar] [CrossRef]
- Shestakova, P.; Martineau, C.; Mavrodinova, V.; Popova, M. Solid state NMR characterization of zeolite beta based drug formulations containing Ag and sulfadiazine. RSC Adv. 2015, 5, 81957. [Google Scholar] [CrossRef]
- Hunger, M. Solid-State NMR Spectroscopy. In Zeolite Characterization and Catalysis; Chester, A.W., Derouane, E.G., Eds.; Springer: Dordrecht, The Netherlands, 2009; Chapter 2; pp. 65–105. [Google Scholar]
- Brunner, E.; Ernst, H.; Freude, D.; Frohlich, T.; Hunger, M.; Pfeifer, H. Magic Angle Spinning NMR Studies of Acid Sites in Zeolite H-ZSM-5. J. Catal. 1991, 127, 34–41. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; Koningsberger, D.C.; Kunkeler, P.; van Bekkum, H.; Kentgens, A.P.M. Stepwise Dealumination of Zeolite Beta at Specific T-Sites Observed with 27Al MAS and 27Al MQ MAS NMR. J. Am. Chem. Soc. 2000, 122, 12842–12847. [Google Scholar] [CrossRef]
- Akçay, M. The surface acidity and characterization of Fe-montmorillonite probed by in situ FT-IR spectroscopy of adsorbed pyridine. Appl. Catal. A Gen. 2005, 294, 156–160. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared Study of Pyridine Adsorbed on Acidic solids. Characterization of Surface Acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Liao, Y.; Zhong, R.; Makshina, E.; d’Halluin, M.; van Limbergen, Y.; Verboekend, D.; Sels, B.F. Propylphenol to Phenol and Propylene over Acidic Zeolites: Role of Shape Selectivity and Presence of Steam. ACS Catal. 2018, 8, 7861–7878. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Massiani, P.; Di Renzo, F.; Espiau, P.; Fajula, F.; Des Courières, T. Study of the state of aluminium in zeolite-β. Appl. Catal. 1991, 72, 139–152. [Google Scholar] [CrossRef]
- Landau, M.V.; Tavor, D.; Regev, O.; Kaliya, M.L.; Herskowitz, M.; Valtchev, V.; Mintova, S. Colloidal nanocrystals of zeolite β stabilized in alumina matrix. Chem. Mater. 1999, 8, 2030–2037. [Google Scholar] [CrossRef]












| Samples | BET Surface Area, (m2/g) | Pore Volume, (cm3/g) | Crystallite Size, (nm) | Reducibility a, (%) |
|---|---|---|---|---|
| Beta | 627 | 0.46 | - | - |
| 10Ni/HB | 577 | 0.34 | 5-NiO | 90 |
| 10Ni1Pt/HB | 584 | 0.36 | 26-NiO | 100 (100) b |
| 1Pt/HB | 604 | 0.42 | ˂5 | 100 b |
| 5Ru/HB | 579 | 0.36 | 26-RuO2 | 96 c |
| 5Ru1Pt/HB | 598 | 0.40 | 29-RuO2 | 100 (100) b |
| Sample | Si(1OH) −101 ppm | Si(1Al) | Si(0Al) | Si/Al | FAl, % | EFAl% | ||
|---|---|---|---|---|---|---|---|---|
| −105 ppm | −107 ppm | −110 ppm | −114 ppm | |||||
| Beta | 19 * | 19 | - | 49 | 13 | 21 | 80 | 20 |
| 10Ni/HB | 9 | 11 | 15 | 59 | 6 | 15 | 95 | 5 |
| 10Ni1Pt/HB | 14 | 17 | 10 | 52 | 7 | 15 | 86 | 14 |
| 1Pt/HB | 6 | 12 | 19 | 54 | 9 | 13 | 81 | 19 |
| 5Ru/HB | 12 | 13 | 15 | 52 | 9 | 14 | 80 | 20 |
| 5Ru1Pt/HB | 15 | 12 | 10 | 55 | 7 | 18 | 83 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, M.; Szegedi, Á.; Oykova, M.; Lazarova, H.; Koseva, N.; Mihályi, M.R.; Karashanova, D.; Mitrev, Y.; Shestakova, P. Hydrodemethoxylation/Dealkylation on Bifunctional Nanosized Zeolite Beta. Molecules 2021, 26, 7694. https://doi.org/10.3390/molecules26247694
Popova M, Szegedi Á, Oykova M, Lazarova H, Koseva N, Mihályi MR, Karashanova D, Mitrev Y, Shestakova P. Hydrodemethoxylation/Dealkylation on Bifunctional Nanosized Zeolite Beta. Molecules. 2021; 26(24):7694. https://doi.org/10.3390/molecules26247694
Chicago/Turabian StylePopova, Margarita, Ágnes Szegedi, Manuela Oykova, Hristina Lazarova, Neli Koseva, Magdolna R. Mihályi, Daniela Karashanova, Yavor Mitrev, and Pavletta Shestakova. 2021. "Hydrodemethoxylation/Dealkylation on Bifunctional Nanosized Zeolite Beta" Molecules 26, no. 24: 7694. https://doi.org/10.3390/molecules26247694