Interactions of β-Lactoglobulin with Bovine Submaxillary Mucin vs. Porcine Gastric Mucin: The Role of Hydrophobic and Hydrophilic Residues as Studied by Fluorescence Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
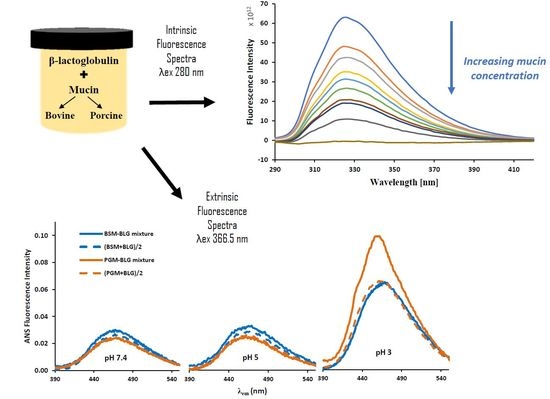
2.1. Effect of Mucins (BSM and PGM) and pH on the Intrinsic Fluorescence Intensity of BLG
2.2. ANS Binding of BLG, BSM, and PGM and Effect of pH on Their ANS Fluorescence Intensity
2.3. Effect of pH and BLG–Mucin Interaction on ANS Binding and ANS Fluorescence Intensity
3. Materials and Methods
3.1. Intrinsic Fluorescence Spectroscopy
3.2. Extrinsic Fluorescence Spectroscopy
3.3. Circular Dichroism
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Busti, P.; Gatti, C.A.; Delorenzi, N.J. Some aspects of β-lactoglobulin structural properties in solution studied by fluorescence quenching. Int. J. Biol. Macromol. 1998, 23, 143–148. [Google Scholar] [CrossRef]
- Sawyer, L.; Kontopidis, G. The core lipocalin, bovine β-lactoglobulin. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2000, 1482, 136–148. [Google Scholar] [CrossRef]
- Sakurai, K.; Konuma, T.; Yagi, M.; Goto, Y. Structural dynamics and folding of β-lactoglobulin probed by heteronuclear NMR. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 527–537. [Google Scholar] [CrossRef]
- Çelebioğlu, H.Y.; Gudjónsdóttir, M.; Meier, S.; Duus, J.Ø.; Lee, S.; Chronakis, I.S. Spectroscopic studies of the interactions between β-lactoglobulin and bovine submaxillary mucin. Food Hydrocoll. 2015, 50, 203–210. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cerbulis, J.; Farrell Jr, H. Composition of milks of dairy cattle. I. Protein, lactose, and fat contents and distribution of protein fraction. J. Dairy Sci. 1975, 58, 817–827. [Google Scholar] [CrossRef]
- McKenzie, H.; Sawyer, W. Effect of p H on β-Lactoglobulins. Nature 1967, 214, 1101–1104. [Google Scholar] [CrossRef]
- Engelhardt, K.; Lexis, M.; Gochev, G.; Konnerth, C.; Miller, R.; Willenbacher, N.; Peukert, W.; Braunschweig, B. pH effects on the molecular structure of β-lactoglobulin modified air–water interfaces and its impact on foam rheology. Langmuir 2013, 29, 11646–11655. [Google Scholar] [CrossRef]
- Fugate, R.D.; Song, P.-S. Spectroscopic characterization of beta-lactoglobulin-retinol complex. Biochim. Biophys. Acta 1980, 625, 28–42. [Google Scholar] [CrossRef]
- Brownlow, S.; Cabral, J.H.M.; Cooper, R.; Flower, D.R.; Yewdall, S.J.; Polikarpov, I.; North, A.C.; Sawyer, L. Bovine β-lactoglobulin at 1.8 Å resolution—still an enigmatic lipocalin. Structure 1997, 5, 481–495. [Google Scholar] [CrossRef] [Green Version]
- Tanford, C.; Nozaki, Y. Physico-chemical comparison of β-lactoglobulins A and B. J. Biol. Chem. 1959, 234, 2874–2877. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Svensson, O.; Arnebrant, T. Mucin layers and multilayers—Physicochemical properties and applications. Curr. Opin. Colloid Interface Sci. 2010, 15, 395–405. [Google Scholar] [CrossRef]
- Lee, S.; Müller, M.; Rezwan, K.; Spencer, N.D. Porcine gastric mucin (PGM) at the water/poly (dimethylsiloxane) (PDMS) interface: Influence of pH and ionic strength on its conformation, adsorption, and aqueous lubrication properties. Langmuir 2005, 21, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.B.; Sotres, J.; Pakkanen, K.I.; Efler, P.; Svensson, B.; Abou Hachem, M.; Arnebrant, T.; Lee, S. Structural and mechanical properties of thin films of bovine submaxillary mucin versus porcine gastric mucin on a hydrophobic surface in aqueous solutions. Langmuir 2016, 32, 9687–9696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakowicz, J.R. Instrumentation for fluorescence spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; pp. 25–61. [Google Scholar]
- Kanakis, C.; Hasni, I.; Bourassa, P.; Tarantilis, P.; Polissiou, M.; Tajmir-Riahi, H.-A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Mensi, A.; Choiset, Y.; Rabesona, H.; Haertlé, T.; Borel, P.; Chobert, J.-M. Interactions of β-lactoglobulin variants A and B with vitamin A. Competitive binding of retinoids and carotenoids. J. Agric. Food Chem. 2013, 61, 4114–4119. [Google Scholar] [CrossRef]
- Liang, L.; Subirade, M. Study of the acid and thermal stability of β-lactoglobulin–ligand complexes using fluorescence quenching. Food Chem. 2012, 132, 2023–2029. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Complexation of bovine β-lactoglobulin with malvidin-3-O-glucoside and its effect on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 209, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Winter, S.; Löber, G. Use of fluorescence decay times of 8-ANS-protein complexes to study the conformational transitions in proteins which unfold through the molten globule state. Biophys. Chem. 1996, 60, 79–88. [Google Scholar] [CrossRef]
- Daniel, E.; Weber, G. Cooperative effects in binding by bovine serum albumin. I. The binding of 1-anilino-8-naphthalenesulfonate. Fluorimetric titrations. Biochemistry 1966, 5, 1893–1900. [Google Scholar] [CrossRef]
- Cattoni, D.I.; Kaufman, S.B.; Flecha, F.L.G. Kinetics and thermodynamics of the interaction of 1-anilino-naphthalene-8-sulfonate with proteins. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- D’Alfonso, L.; Collini, M.; Baldini, G. Evidence of heterogeneous 1-anilinonaphthalene-8-sulfonate binding to β-lactoglobulin from fluorescence spectroscopy. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1999, 1432, 194–202. [Google Scholar] [CrossRef]
- Collini, M.; D’ALFONSO, L.; Baldini, G. New insight on β-lactoglobulin binding sites by 1-anilinonaphthalene-8-sulfonate fluorescence decay. Protein Sci. 2000, 9, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Pasdar, N.; Li-Chan, E.C. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef]
- Santambrogio, C.; Grandori, R. Monitoring the Tanford transition in β-lactoglobulin by 8-anilino-1-naphthalene sulfonate and mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up-to-the-Minute Res. Mass Spectrom. 2008, 22, 4049–4054. [Google Scholar] [CrossRef]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Mateus, N.; de Freitas, V.; Soares, S. Molecular study of mucin-procyanidin interaction by fluorescence quenching and Saturation Transfer Difference (STD)-NMR. Food Chem. 2017, 228, 427–434. [Google Scholar] [CrossRef]
- Celli, G.B.; Liu, Y.; Dadmohammadi, Y.; Tiwari, R.; Raghupathi, K.; Mutilangi, W.; Abbaspourrad, A. Instantaneous interaction of mucin with pectin-and carrageenan-coated nanoemulsions. Food Chem. 2020, 309, 125795. [Google Scholar] [CrossRef]
- Cardamone, M.; Puri, N. Spectrofluorimetric assessment of the surface hydrophobicity of proteins. Biochem. J. 1992, 282, 589–593. [Google Scholar] [CrossRef]
- Mills, O.; Creamer, L. A conformational change in bovine β-lactoglobulin at low pH. Biochim. Biophys. Acta (BBA)-Protein Struct. 1975, 379, 618–626. [Google Scholar] [CrossRef]
- Giraddi, T.; Kadadevarmath, J.; Malimath, G.; Chikkur, G. Effect of solvent on the fluorescence quenching of organic liquid scintillators by aniline and carbon tetrachloride. Appl. Radiat. Isot. 1996, 47, 461–466. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Green, R.J.; Frazier, R.A. Interaction of flavonoids with bovine serum albumin: A fluorescence quenching study. J. Agric. Food Chem. 2005, 53, 158–163. [Google Scholar] [CrossRef]
- Cao, X.; Bansil, R.; Bhaskar, K.R.; Turner, B.S.; LaMont, J.T.; Niu, N.; Afdhal, N.H. pH-dependent conformational change of gastric mucin leads to sol-gel transition. Biophys. J. 1999, 76, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.F.; LaMont, J.T. Hydrophobic binding properties of bovine gallbladder mucin. J. Biol. Chem. 1984, 259, 12170–12177. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Campos-Terán, J.; Hernández-Arana, A.; McClements, D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chem. 2016, 213, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ali, V.; Prakash, K.; Kulkarni, S.; Ahmad, A.; Madhusudan, K.; Bhakuni, V. 8-anilino-1-naphthalene sulfonic acid (ANS) induces folding of acid unfolded cytochrome c to molten globule state as a result of electrostatic interactions. Biochemistry 1999, 38, 13635–13642. [Google Scholar] [CrossRef]
- Çelebioğlu, H.Y.; Gudjónsdóttir, M.; Chronakis, I.S.; Lee, S. Investigation of the interaction between mucins and β-lactoglobulin under tribological stress. Food Hydrocoll. 2016, 54, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of salivary mucins and saliva with food proteins: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 64–83. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz, H.; Lee, S.; Chronakis, I.S. Interactions of β-Lactoglobulin with Bovine Submaxillary Mucin vs. Porcine Gastric Mucin: The Role of Hydrophobic and Hydrophilic Residues as Studied by Fluorescence Spectroscopy. Molecules 2021, 26, 6799. https://doi.org/10.3390/molecules26226799
Yılmaz H, Lee S, Chronakis IS. Interactions of β-Lactoglobulin with Bovine Submaxillary Mucin vs. Porcine Gastric Mucin: The Role of Hydrophobic and Hydrophilic Residues as Studied by Fluorescence Spectroscopy. Molecules. 2021; 26(22):6799. https://doi.org/10.3390/molecules26226799
Chicago/Turabian StyleYılmaz, Hilal, Seunghwan Lee, and Ioannis S. Chronakis. 2021. "Interactions of β-Lactoglobulin with Bovine Submaxillary Mucin vs. Porcine Gastric Mucin: The Role of Hydrophobic and Hydrophilic Residues as Studied by Fluorescence Spectroscopy" Molecules 26, no. 22: 6799. https://doi.org/10.3390/molecules26226799