Diterpenoid Compounds Isolated from Chloranthus oldhamii Solms Exert Anti-Inflammatory Effects by Inhibiting the IKK/NF-κB Pathway
Abstract
:1. Introduction
2. Results
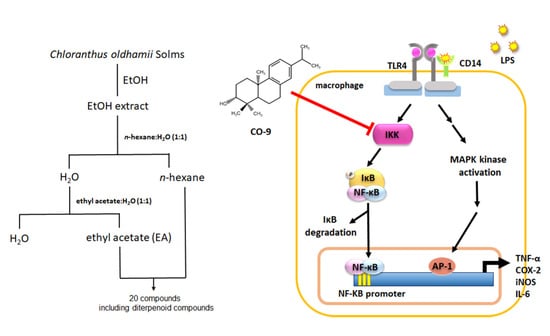
2.1. Isolation and Identification of Anti-Inflammatory Compounds from C. oldhamii
2.2. Anti-Inflammatory Mechanism of Diterpenoid Compounds (CO-9, CO-10, and CO-15) in LPS-Induced Inflammatory Responses
2.3. The Effect of the CO-9 Compound on the LPS-Activated MAPK Pathway in RAW 264.7 Macrophages
2.4. CO-9 Significantly Inhibits IKK-Mediated NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Extraction and Isolation of Compounds from C. oldhamii Solms
4.3. Cell Culture
4.4. Luciferase Reporter Assay
4.5. Cell Viability Assay
4.6. Preparation of Bone Marrow-Derived Macrophages (BMDMs)
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blotting
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-kappaB Signaling Pathway. Kidney Blood Press Res. 2016, 41, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Lee, J.H.; Kim, H.; Park, S.J.; Joe, E.H.; Jou, I. Control of Inflammatory Responses: A New Paradigm for the Treatment of Chronic Neuronal Diseases. Exp. Neurobiol. 2015, 24, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zahringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Widera, D.; Martinez Aguilar, R.; Cottrell, G.S. Toll-like receptor 4 and protease-activated receptor 2 in physiology and pathophysiology of the nervous system: More than just receptor cooperation? Neural Regen Res. 2019, 14, 1196–1201. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benevides, P.J.C.; Sartorelli, P.C.; Kato, M.J. Phenylpropanoids and neolignans from Piper regnellii. Phytochemistry 1999, 52, 339–343. [Google Scholar] [CrossRef]
- Gaia, A.M.; Yamaguchi, L.F.; Jeffrey, C.S.; Kato, M.J. Age-dependent changes from allylphenol to prenylated benzoic acid production in Piper gaudichaudianum Kunth. Phytochemistry 2014, 106, 86–93. [Google Scholar] [CrossRef]
- Miyase, T.; Yamaki, K.; Fukushima, S. Studies on Sesquiterpenes from Macroclinidium trilobum MAKINO. I. Chem. Pharm. Bull. 1984, 32, 3912–3917. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.C.; Chen, S.H.; Lin, L.C.; Fu, S.L. Anti-inflammatory Principles from Sarcandra glabra. J. Agric. Food Chem. 2017, 65, 6497–6505. [Google Scholar] [CrossRef]
- Dekebo, A.; Dagne, E.; Sterner, O. Furanosesquiterpenes from Commiphora sphaerocarpa and related adulterants of true myrrh. Fitoterapia 2002, 73, 48–55. [Google Scholar] [CrossRef]
- Heinz Brieskorn, C.; Noble, P. Furanosesquiterpenes from the essential oil of myrrh. Phytochemistry 1983, 22, 1207–1211. [Google Scholar] [CrossRef]
- Anthonsen, T.; Bergland, G.; Lindberg, B.; Svensson, S.; Leander, K.; Swahn, C.-G. Constituents of Solidago Species. III. The Constitution and Stereochemistry of Diterpenoids from Solidago missouriensis Nutt. Acta Chem. Scand. 1973, 27, 1073–1082. [Google Scholar] [CrossRef]
- Seca, A.M.; Silva, A.M.; Bazzocchi, I.L.; Jimenez, I.A. Diterpene constituents of leaves from Juniperus brevifolia. Phytochemistry 2008, 69, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Xiong, J.; Lau, C.; Pan, L.L.; Hu, J.F. Sesquiterpenoids and further diterpenoids from the rare Chloranthaceae plant Chloranthus sessilifolius. J. Asian Nat. Prod. Res. 2015, 17, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, Y.; Yoshikoshi, A.; Oida, S. Total synthesis of dolabradiene. Tetrahedron Lett. 1964, 5, 1763–1770. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Hisada, S.; Nishibe, S. Coumarins from Bark of Fraxinus japonica and F. mandshurica var. japonica. Chem. Pharm. Bull. 1985, 33, 4069–4073. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, J.; Mizutani, J. Dimeric sesquiterpenoid esters from Chloranthus serratus. Phytochemistry 1992, 31, 1293–1296. [Google Scholar] [CrossRef]
- Urones, J.G.; Marcos, I.S.; Ferreras, J.F.N.; Barcala, P.B. Terpenoids from Nepeta tuberosa subsp.reticulata (II). Phytochemistry 1988, 27, 523–526. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.Y.; Satyanandamurty, T.; Wu, J. New diterpenes from a Godavari mangrove, Ceriops decandra. Planta Med. 2013, 79, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Hong, Z.L.; Xu, P.; Zou, Y.; Yu, S.B.; Yang, G.X.; Hu, J.F. ent-Abietane diterpenoids with anti-neuroinflammatory activity from the rare Chloranthaceae plant Chloranthus oldhamii. Org. Biomol. Chem. 2016, 14, 4678–4689. [Google Scholar] [CrossRef]
- Xiong, J.; Hong, Z.L.; Gao, L.X.; Shen, J.; Liu, S.T.; Yang, G.X.; Li, J.; Zeng, H.; Hu, J.F. Chlorabietols A-C, Phloroglucinol-Diterpene Adducts from the Chloranthaceae Plant Chloranthus oldhamii. J. Org. Chem. 2015, 80, 11080–11085. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Du, Y.; Yin, C.; Suo, X.; Wang, R.; Xia, R.; Zhang, X. Water-separated part of Chloranthus serratus alleviates lipopolysaccharide- induced RAW264.7 cell injury mainly by regulating the MAPK and Nrf2/HO-1 inflammatory pathways. BMC Complement Altern. Med. 2019, 19, 343. [Google Scholar] [CrossRef] [PubMed]
- Chloranthus oldhami Solms. Available online: https://taieol.tw/pages/43420 (accessed on 18 October 2021).
- Hsu, Y.H.; Fu, S.L. Detection of Endotoxin Contamination in Chinese Herbs by NF-kB Activity-Based Reporter Assays. J. Food Drug Anal. 2004, 12, 34–39. [Google Scholar]
- Trouplin, V.; Boucherit, N.; Gorvel, L.; Conti, F.; Mottola, G.; Ghigo, E. Bone marrow-derived macrophage production. J Vis. Exp. 2013, 81, e50966. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, L.-C.; Wang, J.-Y.; Lin, C.-H.; Hsu, C.-H.; Lin, L.-C.; Fu, S.-L. Diterpenoid Compounds Isolated from Chloranthus oldhamii Solms Exert Anti-Inflammatory Effects by Inhibiting the IKK/NF-κB Pathway. Molecules 2021, 26, 6540. https://doi.org/10.3390/molecules26216540
Chiu L-C, Wang J-Y, Lin C-H, Hsu C-H, Lin L-C, Fu S-L. Diterpenoid Compounds Isolated from Chloranthus oldhamii Solms Exert Anti-Inflammatory Effects by Inhibiting the IKK/NF-κB Pathway. Molecules. 2021; 26(21):6540. https://doi.org/10.3390/molecules26216540
Chicago/Turabian StyleChiu, Lin-Chieh, Jir-You Wang, Chao-Hsiung Lin, Chung-Hua Hsu, Lie-Chwen Lin, and Shu-Ling Fu. 2021. "Diterpenoid Compounds Isolated from Chloranthus oldhamii Solms Exert Anti-Inflammatory Effects by Inhibiting the IKK/NF-κB Pathway" Molecules 26, no. 21: 6540. https://doi.org/10.3390/molecules26216540