Design and Antioxidant Properties of Bifunctional 2H-Imidazole-Derived Phenolic Compounds—A New Family of Effective Inhibitors for Oxidative Stress-Associated Destructive Processes
Abstract
:1. Introduction
2. Results
2.1. Study of Electrochemical Behavior of Synthesized Compounds
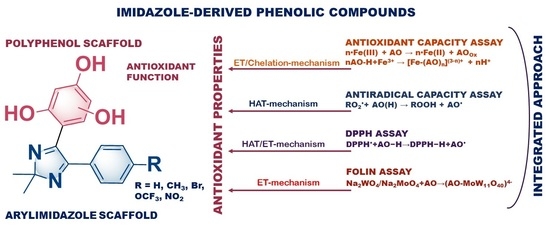
2.2. Study of Antioxidant Properties of Polyphenols and Aynthesized Compounds
- Electron-transfer (ET);
- Hydrogen atom transfer (HAT);
- Metal chelating mechanism.
2.3. Evaluation of the Reaction Half-Life (t1/2) by Using Potentiometric Method
3. Materials and Methods
3.1. Apparatus
3.2. Reagents and Chemicals
3.3. Experimental
3.3.1. General Procedure for the Synthesis of Hydrochloride Salt of Imidazole-Derived Polyphenolic Compounds Im(1–5)Phl, Im5Pyr, Im5Hyd
3.3.2. General Procedure for the Synthesis of 2H-imidazoles Im1–2
3.4. Procedures
3.4.1. Cyclic Voltammetry
3.4.2. Potentiometric Assay of Determining Antioxidant Capacity
3.5. Potentiometric Assay of Determining Antiradical Capacity
3.6. Folin Assay
3.7. DPPH Assay
3.8. Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Menshchikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress. Pathologic States and Diseases; Sibirskoe Univ. Izd.: Novosibirsk, Russia, 2008; p. 534. [Google Scholar]
- Menschikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress. Pathological Conditions and Diseases; ARTA: Novosibirsk, Russia, 2008; p. 435. [Google Scholar]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef]
- Hojs, N.V.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef]
- Andrisic, L.; Dudzik, D.; Barbas, C.; Milkovic, L.; Grune, T.; Zarkovic, N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018, 14, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, W.; Chen, X.H.; Wang, S.B.; Qian, W.B. Oxidative stress response induced by chemotherapy in leukemia treatment. Mol. Clin. Oncol. 2018, 8, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.H.; Al-ataby, I.A.; Mahmod, A.I.; Jawarneh, S.; Al Kury, L.T.; Al-Yasari, I.H. The Impact of Herbal Infusion Consumption on Oxidative Stress and Cancer: The Good, the Bad, the Misunderstood. Molecules 2020, 25, 4207. [Google Scholar] [CrossRef]
- Cebova, M.; Pechanova, O. Protective Effects of Polyphenols against Ischemia/Reperfusion Injury. Molecules 2020, 25, 3469. [Google Scholar] [CrossRef]
- Rosenbaugh, E.G.; Savalia, K.K.; Manickam, D.S.; Zimmerman, M.C. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R917–R928. [Google Scholar] [CrossRef]
- Alsulaimani, M.A.; Magadmi, R.M.; Esmat, A. Mechanisms of Diabetic Neuropathies and Antioxidant Therapy. J. Pharm. Res. Int. 2020, 32, 28–43. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Zavala-Flores, L.; Anandhan, A.; Wang, F.; Skotak, M.; Chandra, N.; Li, M.; Pappa, A.; Martinez-Fong, D.; Del Razo, L.M.; et al. Antioxidant gene therapy against neuronal cell death. Pharmacol. Ther. 2014, 142, 206–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baunthiyal, M.; Singh, V.; Dwivedi, S. Insights of Antioxidants as Molecules for Drug Discovery. Int. J. Pharmacol. 2017, 13, 874–889. [Google Scholar] [CrossRef] [Green Version]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Popov, A.F.; Shchelkanov, M.Y.; Dmitrenko, K.A.; Simakova, A.I. Combined therapy of influenza with antiviral drugs with a different mechanism of action in comparison with monotherapy. J. Pharm. Sci. Res. 2018, 10, 357–360. [Google Scholar]
- Pritchard, J.R.; Bruno, P.M.; Gilbert, L.A.; Capron, K.L.; Lauffenburger, D.A.; Hemann, M.T. Defining principles of combination drug mechanisms of action. Proc. Natl. Acad. Sci. USA 2013, 110, E170–E179. [Google Scholar] [CrossRef] [Green Version]
- Jeřábek, J.; Uliassi, E.; Guidotti, L.; Korábečný, J.; Soukup, O.; Sepsova, V.; Hrabinova, M.; Kuča, K.; Bartolini, M.; Peña-Altamira, L.E.; et al. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 250–262. [Google Scholar] [CrossRef]
- Jones, M.R.; Mathieu, E.; Dyrager, C.; Faissner, S.; Vaillancourt, Z.; Korshavn, K.J.; Lim, M.H.; Ramamoorthy, A.; Yong, V.W.; Tsutsui, S.; et al. Multi-target-directed phenol–triazole ligands as therapeutic agents for Alzheimer’s disease. Chem. Sci. 2017, 8, 5636–5643. [Google Scholar] [CrossRef] [Green Version]
- Shalmali, N.; Ali, M.R.; Bawa, S. Imidazole: An Essential Edifice for the Identification of New Lead Compounds and Drug Development. Mini Rev. Med. Chem. 2018, 18, 142–163. [Google Scholar] [CrossRef]
- Mumtaz, A.; Saeed, A.; Fatima, N.; Dawood, M.; Rafique, H.; Iqbal, J. Imidazole and its derivatives as potential candidates for drug development. Bangladesh J. Pharmacol. 2016, 11, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Dake, S.A.; Shinde, S.V.; Vyawahare, S.K.; Marathe, R.P.; Navale, R.B.; Sawale, A.R.; Pawar, R.P. Bioactivity and synthesis of substituted imidazole motifs. In Bioactive Heterocycles: Synthesis and Biological Evaluation; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 69–96. [Google Scholar]
- Nilotinib. DrugBank ID DB04868. Available online: https://www.drugbank.ca/drugs/DB04868 (accessed on 2 August 2021).
- Telmisartan. DrugBank ID DB00966. Patent US8003679, 23 August 2011. Available online: https://www.drugbank.ca/drugs/DB00966 (accessed on 2 August 2021).
- Selumetinib. DrugBank ID DB11689. Patent US9156795, 13 October 2015. Available online: https://www.drugbank.ca/drugs/DB11689 (accessed on 2 August 2021).
- Selumetinib. DrugBank ID DB00678. Patent CA2085584, 2 November 2003. Available online: https://www.drugbank.ca/drugs/DB00678 (accessed on 2 August 2021).
- Soto-Hernndez, M.; Palma-Tenango, M.; del Rosario Garcia-Mateos, M. (Eds.) Phenolic Compounds—Biological Activity; InTech: West Palm Beach, FL, USA, 2017; ISBN 978-953-51-2959-2. [Google Scholar]
- Carocho, M.; Ferreira, I. The Role of Phenolic Compounds in the Fight against Cancer—A Review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-Oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, P.; Li, X.; Song, T.; Chai, G.; Yu, X.; Zhang, K. Novel Mcl-1/Bcl-2 Dual Inhibitors Created by the Structure-Based Hybridization of Drug-Divided Building Blocks and a Fragment Deconstructed from a Known Two-Face BH3 Mimetic. Arch. Pharm. Chem. Life Sci. 2015, 348, 89–99. [Google Scholar] [CrossRef]
- Matysiak, J.; Skrzypek, A.; Karpińska, M.; Czarnecka, K.; Szymański, P.; Bajda, M.; Niewiadomy, A. Biological Evaluation, Molecular Docking, and SAR Studies of Novel 2-(2,4-Dihydroxyphenyl)-1H- Benzimidazole Analogues. Biomolecules 2019, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, S.; Yang, S.; Guo, X.; Lou, H.; Ren, D. Phenolic Alkaloids from the Aerial Parts of Dracocephalum Heterophyllum. Phytochemistry 2012, 82, 166–171. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley-Blackwell: England, UK, 2018; p. 337. [Google Scholar]
- Apak, R.; Ozyurek, M.; Guklu, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guklu, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. An integrated approach to the investigation of antioxidant properties by potentiometry. Anal. Chim. Acta 2020, 1111, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E.; Borisova, M.; Drokin, R.; Gorbunov, E.; Ulomskiy, E.; Rusinov, V. The antioxidant screening of potential materials for drugs based on 6-nitro-1,2,4-triazoloazines containing natural polyphenol fragments. Anal. Bioanal. Chem. 2020, 412, 5147–5155. [Google Scholar] [CrossRef]
- Gaikwad, P.; Barik, A.; Priyadarsini, K.I.; Rao, B.S.M. Antioxidant activities of phenols in different solvents using DPPH assay. Res. Chem. Intermed. 2010, 36, 1065–1072. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Maciel, L.G.; Nunes, D.S. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. Trends Anal. Chem. 2016, 80, 266–279. [Google Scholar] [CrossRef]
- Moseev, T.D.; Nikiforov, E.A.; Varaksin, M.V.; Charushin, V.N.; Chupakhin, O.N. Metal-Free C–H/C–H Coupling of 2H-Imidazole 1-Oxides with Polyphenols towards Imidazole-Linked Polyphenolic Compounds. J. Org. Chem. 2021, 86, 13702–13710. [Google Scholar] [CrossRef]
- Akulov, A.A.; Varaksin, M.V.; Charushin, V.N.; Chupakhin, O.N. C(sp2)-H functionalization of aldimines and related compounds: Advances and prospects. Russ. Chem. Rev. 2021, 90, 374–394. [Google Scholar] [CrossRef]
- Akulov, A.A.; Varaksin, M.V.; Mampuys, P.; Charushin, V.N.; Chupakhin, O.N.; Maes, B.U.W. C(sp2)–H functionalization in non-aromatic azomethine-based heterocycles. Org. Biomol. Chem. 2021, 19, 297–312. [Google Scholar] [CrossRef]
- Moseev, T.D.; Varaksin, M.V.; Gorlov, D.A.; Charushin, V.N.; Chupakhin, O.N. Transition-Metal-Free C–H/C–Li Coupling of Nonaromatic 2H-Imidazole 1-Oxides with Pentafluorophenyl Lithium in the Design of Novel Fluorophores with Intramolecular Charge Transfer Effect. J. Org. Chem. 2020, 85, 11124–11133. [Google Scholar] [CrossRef] [PubMed]
- Smyshliaeva, L.A.; Varaksin, M.V.; Slepukhin, P.A.; Chupakhin, O.N.; Charushin, V.N. Transition metal-free oxidative and deoxygenative C–H/C–Li cross-couplings of 2H-imidazole 1-oxides with carboranyl lithium as an efficient synthetic approach to azaheterocyclic carboranes. Beilstein J. Org. Chem. 2018, 14, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Varaksin, M.; Moseev, T.; Chupakhin, O.; Charushin, V.; Trofimov, B. Metal-free C–H functionalization of 2H-imidazole 1-oxides with pyrrolyl fragments in the design of novel azaheterocyclic ensembles. Org. Biomol. Chem. 2017, 15, 8280–8284. [Google Scholar] [CrossRef] [PubMed]
- Akulov, A.A.; Varaksin, M.V.; Tsmokalyuk, A.N.; Charushin, V.N.; Chupakhin, O.N. Blue-light-promoted radical C–H azolation of cyclic nitrones enabled by Selectfluor®. Green Chem. 2021, 23, 2049–2057. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Archana, I.; Vijayalakshmi, K. Antioxidant potential of phloroglucinol; An in vitro approach. Int. J. Pharm. Sci. Res. 2018, 9, 2947–2951. [Google Scholar]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Waldron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–193. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Gerasimova, E.; Gazizullina, E.; Radosteva, E.; Ivanova, A. Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies. Chemosensors 2021, 9, 112. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. New antiradical capacity assay with the use potentiometric method. Anal. Chim. Acta 2019, 1046, 69–76. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Kirilyuk, I.A.; Grigor’ev, I.A.; Volodarskii, L.B. Synthesis of 2H-imidazole 1-oxides and stable nitroxyl radicals based on them. Bull. Acad. Sci. USSR Div. Chem. Sci. 1991, 40, 1871–1879. [Google Scholar] [CrossRef]







| Polyphenol Scaffold Imidazole Scaffold |  |  |  |  |
|---|---|---|---|---|
 | Phl a | Pyr a | Hyd a | |
 | Im1H (72%) | Im1Phl (94%) | Im1Pyr (85%) b | Im1Hyd (93%) b |
 | Im2H (80%) | Im2Phl (95%) | Im2Pyr (75%) b | Im2Hyd (88%) b |
 | - | Im3Phl (92%) | Im3Pyr (90%) b | Im3Hyd (91%) b |
 | - | Im4Phl (87%) | Im4Pyr (87%) b | Im4Hyd (90%) b |
 | - | Im5Phl (88%) | Im5Pyr (91%) | Im5Hyd (93%) |
| Polyphenol | Imidazolylpolyphenols | Eox, V | iox, 106 A |
|---|---|---|---|
| Phloroglucinol | Phl | 0.64 | 2.80 |
| Im1Phl | 1.02 | 3.27 | |
| Im2Phl | 1.02 | 1.85 | |
| Im3Phl | 0.99 | 4.69 | |
| Im4Phl | 1.02 | 4.45 | |
| Im5Phl | 0.98 | 5.09 | |
| Pyrogallol | Pyr | 0.18 0.60 | 2.90 1.63 |
| Im1Pyr | 0.57 1.09 | 6.61 1.93 | |
| Im2Pyr | 0.45 1.00 | 8.78 3.10 | |
| Im3Pyr | 0.45 0.98 | 7.82 3.23 | |
| Im4Pyr | 0.52 1.03 | 9.35 3.51 | |
| Im5Pyr | 0.18 0.64 | 5.59 7.00 | |
| Hydroxyquinol | Hyd | −0.02 1.08 | 9.80 2.20 |
| Im1Hyd | 0.36 1.11 | 10.41 11.41 | |
| Im2Hyd | 0.54 1.20 | 7.26 6.64 | |
| Im3Hyd | 0.38 1.12 | 8.62 7.97 | |
| Im4Hyd | 0.38 1.15 | 14.40 12.37 | |
| Im5Hyd | 0.14 1.20 | 1.27 6.46 |
| Substrates | AOC | ARC | Folin Assay | DPPH Assay | ||||
|---|---|---|---|---|---|---|---|---|
| 104 mol-eq/dm3 | RSD (%) | 104 mol-eq/dm3 | RSD (%) | C(GA) 104 mol-eq/dm3 | RSD (%) | C(AA) 104 mol-eq/dm3 | RSD (%) | |
| Phl | 2.84 ± 0.23 | 8.1 | 2.26 ± 0.04 | 2.2 | - | - | 1.22 ± 0.02 | 1.4 |
| Pyr | 5.15 ± 0.11 | 1.9 | 2.84 ± 0.03 | 3.2 | 2.08 ± 0.02 | 0.9 | 1.47 ± 0.03 | 1.2 |
| Hyd | 1.92 ± 0.04 | 1.9 | 1.89 ± 0.02 | 0.9 | 0.74 ± 0.01 | 1.3 | 1.40 ± 0.01 | 1.5 |
| Im1Phl | 1.95 ± 0.04 | 1.9 | 4.01 ± 0.08 | 1.9 | - | - | 0.78 ± 0.01 | 1.4 |
| Im2Phl | 2.74 ± 0.08 | 3.1 | 4.02 ± 0.16 | 3.8 | - | - | 0.71 ± 0.03 | 3.3 |
| Im3Phl | 1.89 ± 0.02 | 0.9 | * | - | - | - | - | |
| Im4Phl | 1.87 ± 0.04 | 2.1 | * | - | - | 0.69 ± 0.01 | 2.4 | |
| Im5Phl | 1.88 ± 0.08 | 4.1 | 5.16 ± 0.17 | 2.9 | - | - | 0.70 ± 0.02 | 2.1 |
| Im1Pyr | 2.24 ± 0.09 | 3.8 | 2.15 ± 0.02 | 0.9 | 0.50 ± 0.05 | 9.1 | 1.17 ± 0.02 | 2.1 |
| Im2Pyr | 2.46 ± 0.02 | 1.2 | 1.51 ± 0.02 | 1.9 | 0.44 ± 0.004 | 1.1 | 1.17 ± 0.01 | 1.2 |
| Im3Pyr | 1.76 ± 0.07 | 3.9 | 2.52 ± 0.07 | 3.2 | 0.40 ± 0.02 | 5.5 | 0.93 ± 0.04 | 4.2 |
| Im4Pyr | 1.94 ± 0.04 | 1.7 | 2.97 ± 0.03 | 0.8 | 0.41 ± 0.03 | 7.9 | 1.22 ± 0.01 | 1.1 |
| Im5Pyr | 4.19 ± 0.34 | 7.8 | 1.26 ± 0.06 | 4.7 | 0.61 ± 0.07 | 10.6 | 1.22 ± 0.01 | 1.1 |
| Im1Hyd | 0.98 ± 0.03 | 2.7 | 0.41 ± 0.01 | 1.6 | 0.68 ± 0.05 | 6.9 | 1.12 ± 0.02 | 1.9 |
| Im2Hyd | 0.75 ± 0.05 | 6.7 | 0.40 ± 0.02 | 3.8 | 0.78 ± 0.05 | 6.1 | 1.06 ± 0.01 | 1.3 |
| Im3Hyd | 0.72 ± 0.01 | 1.7 | 0.81 ± 0.02 | 2.9 | 1.15 ± 0.06 | 5.1 | 1.12 ± 0.07 | 5.9 |
| Im4Hyd | 1.58 ± 0.03 | 1.8 | 1.13 ± 0.06 | 4.6 | 0.75 ± 0.09 | 11.0 | 0.95 ± 0.02 | 1.9 |
| Im5Hyd | 2.78 ± 0.25 | 8.8 | 0.34 ± 0.06 | 2.3 | 0.92 ± 0.09 | 10.1 | 1.22 ± 0.00 | 0 |
| Substance | t1/2 (s) | RSD (%) |
|---|---|---|
| Phloroglucinol | 133 ± 6 | 4.8 |
| Pyrogallol | 5.2 ± 0.3 | 4.6 |
| Hydroxyquinol | 1.3 ± 0.1 | 7.6 |
| Im1Phl | 42 ± 2 | 4.7 |
| Im2Phl | 86 ± 3 | 4.0 |
| Im3Phl | 52 ± 2 | 4.6 |
| Im4Phl | 46 ± 3 | 8.1 |
| Im5Phl | 73 ± 7 | 9.2 |
| Im1Pyr | 9.2 ± 0.3 | 2.4 |
| Im2Pyr | 5.5 ± 0.4 | 6.9 |
| Im3Pyr | 15.3 ± 0.7 | 4.7 |
| Im4Pyr | 7.2 ± 0.1 | 3.0 |
| Im5Pyr | 11.3 ± 0.64 | 5.9 |
| Im1Hyd | 4.5 ± 0.2 | 4.2 |
| Im2Hyd | 6.4 ± 0.2 | 3.9 |
| Im3Hyd | 5.5 ± 0.3 | 4.7 |
| Im4Hyd | 11.1 ± 0.5 | 4.8 |
| Im5Hyd | 4.5 ± 0.1 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, E.L.; Gazizullina, E.R.; Borisova, M.V.; Igdisanova, D.I.; Nikiforov, E.A.; Moseev, T.D.; Varaksin, M.V.; Chupakhin, O.N.; Charushin, V.N.; Ivanova, A.V. Design and Antioxidant Properties of Bifunctional 2H-Imidazole-Derived Phenolic Compounds—A New Family of Effective Inhibitors for Oxidative Stress-Associated Destructive Processes. Molecules 2021, 26, 6534. https://doi.org/10.3390/molecules26216534
Gerasimova EL, Gazizullina ER, Borisova MV, Igdisanova DI, Nikiforov EA, Moseev TD, Varaksin MV, Chupakhin ON, Charushin VN, Ivanova AV. Design and Antioxidant Properties of Bifunctional 2H-Imidazole-Derived Phenolic Compounds—A New Family of Effective Inhibitors for Oxidative Stress-Associated Destructive Processes. Molecules. 2021; 26(21):6534. https://doi.org/10.3390/molecules26216534
Chicago/Turabian StyleGerasimova, Elena L., Elena R. Gazizullina, Maria V. Borisova, Dinara I. Igdisanova, Egor A. Nikiforov, Timofey D. Moseev, Mikhail V. Varaksin, Oleg N. Chupakhin, Valery N. Charushin, and Alla V. Ivanova. 2021. "Design and Antioxidant Properties of Bifunctional 2H-Imidazole-Derived Phenolic Compounds—A New Family of Effective Inhibitors for Oxidative Stress-Associated Destructive Processes" Molecules 26, no. 21: 6534. https://doi.org/10.3390/molecules26216534