Analysis of Biogenic Amines Using Immunoassays, HPLC, and a Newly Developed IC-MS/MS Technique in Fish Products—A Comparative Study
Abstract
:1. Introduction
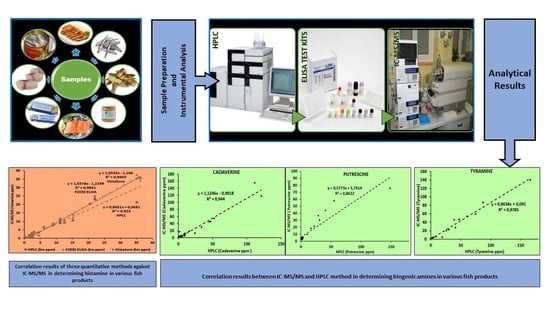
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Sample Preparation
3.4. IC-MS/MS Procedure
3.5. HPLC Procedure and Derivatization Step
3.6. ELISA Procedures
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Paleologos, E.K.; Chytiri, S.D.; Savvaidis, I.N.; Kontominas, M.G. Determination of biogenic amines as their benzoyl derivatives after cloud point extraction with micellar liquid chromatographic separation. J. Chromatogr. A 2003, 1010, 217–224. [Google Scholar] [CrossRef]
- Köse, S. Biogenic Amines. In Toxins and Other Harmful Compounds in Foods; Witczak, A., Sikorski, Z., Eds.; CRC Press: London, UK, 2017; pp. 85–152. [Google Scholar]
- European Commission. RASFF Portal. Available online: https://webgate.ec.europa.eu/rasff-window/portal/?event=searchResultList&StartRow=1 (accessed on 18 December 2020).
- Shalaby, A.R. Significance of biogenic amines in food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- EC Directive, L 338/12 EN Official Journal of the European Union. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 2 July 2021).
- EC. Commission Regulation (EU) No 1019/2013 of 23 October 2013 Amending Annex I to Regulation (EC) No 2073/2005 as Regards Histamine. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/%20PDF/?uri=CELEX:32013R1019&from=EN (accessed on 15 July 2021).
- FAO-WHO. Joint FAO/WHO Expert Meeting on the Public Health Risks of Histamine and Other Biogenic Amines from Fish and Fishery Products. Available online: http://www.fao.org/3/i3390e/i3390e.pdf (accessed on 1 June 2021).
- Hungerford, J.M. Histamine and scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef] [PubMed]
- FDA. Fish and Fisheries Products Hazards and Controls Guide. Available online: http://www.cfsan.fda.gov/*comm/haccp4x5.html (accessed on 21 June 2019).
- Debeer, J.; Bell, J.W.; Nolte, F.; Arcieri, J.; Correa, G. Histamine limits by country: A survey and review. J. Food Prot. 2021, 84, 1610–1628. [Google Scholar] [CrossRef]
- Cinquina, A.L.; Calì, A.; Longo, F.; De Santis, L.; Severoni, A.; Abballe, F. Determination of biogenic amines in fish tissues by ion-exchange chromatography with conductivity detection. J. Chromatogr. A 2004, 1032, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Santos, M. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Özogul, F.; Özogul, Y. Biogenic amine content and biogenic amine quality indices of sardines (Sardina pilchardus) stored in modified atmosphere packing and vacuum packing. Food Chem. 2006, 99, 574–578. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Erim, F.B. Recent analytical approaches to the analysis of biogenic amines in food samples. Trends Anal. Chem. 2013, 52, 239–247. [Google Scholar] [CrossRef]
- Önal, A.; Tekkeli, S.E.K.; Önal, C. A review of the liquid chromatographic methods for the determination of biogenic amines in foods. Food Chem. 2013, 138, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, Z.; Kos, J. Detection of Metabolites of Microbial Origin in Beverages with Harmful Effect on Human Health—Biogenic Amines and Mycotoxins. In Safety Issues in Beverage Production; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 39–77. [Google Scholar]
- Ahangari, H.; Kurbanoglu, S.; Ehsani, A.; Uslu, B. Latest trends for biogenic amines detection in foods: Enzymatic biosensors and nanozymes applications. Trends Food Sci. Technol. 2021, 112, 75–87. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. TrAC Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Rogers, P.L.; Staruszkiewicz, W.F. Histamine test kit comparison. J. Aquat. Food Prod. Technol. 2000, 9, 5–17. [Google Scholar] [CrossRef]
- Muscarella, M.; Iammarino, M.; Centonze, D.; Palermo, C. Measurement of histamine in seafood by HPLC, CE, and ELISA: Comparison of three techniques. Vet. Res. Commun. 2005, 29, 343–346. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Köse, S.; Kaklikkaya, N.; Koral, S.; Tufan, B.; Buruk, C.K.; Aydin, F. Commercial test kits and the determination of histamine in traditional (ethnic) fish products-evaluation against an EU accepted HPLC method. Food Chem. 2011, 125, 1490–1497. [Google Scholar] [CrossRef]
- Hungerford, J.M.; Wu, W.-H. Comparison study of three rapid test kits for histamine in fish: BiooScientific MaxSignal enzymatic assay, Neogen Veratox ELISA, and the Neogen Reveal Histamine Screening Test. Food Control 2012, 25, 448–457. [Google Scholar] [CrossRef]
- Stroka, J.; Bouten, K.; Mischke, C.; Breidbach, A.; Ulberth, F. Equivalence Testing of Histamine Methods—Final Report. Available online: https://op.europa.eu/en/publication-detail/-/publication/de1f7599-a627-4b5e-a24b-d09911b93dbf/language-en (accessed on 23 July 2021).
- Savničar, A.; Rogelj, I.; Kočar, D.; Köse, S.; Pompe, M. Determination of biogenic amines in cheese by ion chromatography with tandem mass spectrometry detection. J. AOAC Int. 2018, 101, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Kočar, D.; Köse, S.; Tufan, B.; Ščavničar, A.; Pompe, M. Determination of biogenic amines in fresh fish and processed fish products using IC-MS/MS. Foods 2021, 10, 1746. [Google Scholar] [CrossRef] [PubMed]
- Duflos, G.; Inglebert, G.; Himber, C.; Degremont, S.; Lombard, B.; Brisabois, A. Validation of standard method EN ISO 19343 for the detection and quantification of histamine in fish and fishery products using high-performance liquid chromatography. Int. J. Food Microbiol. 2019, 288, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Tobeña, A.; Dueñas, S.; Boix, M. Validation study of BioSystems® Y15 Histamine Dehydrogenase Kit for the detection of histamine in fish and fishery products: AOAC Performance Tested MethodSM. J. AOAC Int. 2021, 104, 693–711. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2010. [Google Scholar]
- Paul, J.; Mozola, M. Validation study of a rapid ELISA for detection of histamine in tuna. J. AOAC Int. 2011, 94, 886–899. [Google Scholar]
- Manz, G.; Booltink, E. Validation study of a HistaSure™ ELISA (Fast Track) for the determination of histamine in fish samples. J. AOAC Int. 2014, 97, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Quan, Z.; Evens, J.L.; Byrd, E.A.; Liu, Y.M. Enhancing capillary liquid chromatography/tandem mass spectrometry of biogenic amines by pre-column derivatization with 7-fluoro-4-nitrobenzoxadiazole. Rapid Commun. Mass Spectrom. 2004, 18, 989–994. [Google Scholar] [CrossRef]
- Calbiani, F.; Careri, M.; Elviri, L.; Mangia, A.; Pistarà, L.; Zagnoni, I. Rapid assay for analyzing biogenic amines in cheese: Matrix solid-phase dispersion followed by liquid chromatography-electrospray-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3779–3783. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Gianotti, V.; Polati, S.; Gennaro, M.C. High performance liquid chromatography/tandem mass spectrometry determination of biogenic amines in typical Piedmont cheeses. J. Chromatogr. A 2007, 1149, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Saccani, G.; Tanzi, E.; Pastore, P.; Cavalli, S.; Rey, M. Determination of biogenic amines in fresh and processed meat by suppressed ion chromatography-mass spectrometry using a cation-exchange column. J. Chromatogr. A 2005, 1082, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Millán, S.; Sampedro, M.C.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Simple and rapid determination of biogenic amines in wine by liquid chromatography—Electrospray ionization ion trap mass spectrometry. Anal. Chim. Acta 2007, 584, 145–152. [Google Scholar] [CrossRef]
- Shimoji, K.; Bakke, M. Validation study of histamine test for the determination of histamine in selected fish products. AOAC Performance Tested Method SM 041802. J. AOAC Int. 2019, 102, 164–180. [Google Scholar] [CrossRef]
- Labor Diagnostika Nord. Available online: https://ldn.de/ (accessed on 15 July 2021).
- Booltink, E.; (LDN (Labor Centrum Nordhorn), Nordhorn, Germany). Personal communication, 2021.
- Köse, S.; Koral, S.; Tufan, B.; Pompe, M.; Scavnicar, A.; Kocar, A. Biogenic amine contents of commercially processed traditional fish products originating from European countries and Turkey. Eur. Food Res. Technol. 2012, 235, 669–683. [Google Scholar] [CrossRef]
- Halàsz, A.; Baràth, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Prester, L. Biogenic amines in fish, fish products and shellfish: A review. Food Addit. Contam. Part A 2011, 28, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Mietz, J.L.; Karmas, E. Chemical index of canned tuna determined by high-pressure liquid chromatography. J. Food Sci. 1977, 42, 155–158. [Google Scholar] [CrossRef]
- Veciana-Nogués, M.T.; Marineé-Font, A.; Vidal-Carou, M.C. Biogenic amines as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines and organoleptic changes. J. Agric. Food Chem. 1997, 45, 2036–2041. [Google Scholar] [CrossRef]
- Koral, S.; Tufan, B.; Ščavničar, A.; Kočar, D.; Pompe, M.; Köse, S. Investigation of the contents of biogenic amines and some food safety parameters of various commercially salted fish products. Food Control 2013, 32, 597–606. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Introduction to Biostatistics, 2nd ed.; W.H. Freeman and Co.: New York, NY, USA, 1987. [Google Scholar]




| Quantitative Methods | Semi-Quantitative Methods | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| (Histamine mg/kg) | New HistaSure-Lateral Flow | Old HistaSure–HISTAGOLD | |||||||
| No. | Samples | IC-MS/MS | HPLC | FOOD ELISA | HistaSure ELISA, Fast Track | R | Cut-Off Values | R | Cut-Off Values |
| 1 | Anchovy, Fresh-1 | 4.51 ± 0.67 a | 3.45 ± 0.02 b | 1.81 ± 0.80 c | 1.61 ± 0.20 c | N | 10 mg/kg | N | 5, 25, 50 mg/kg |
| 2 | Anchovy, Fresh-2 | 1.98 ± 0.21 a | 1.97 ± 0.42 a | <0.75 b | <0.75 b | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 3 | Anchovy, Fresh-3 | NA | 2.14 ± 0.09 a | <0.75 b | <0.75 b | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 4 | Anchovy, Brined-1 (15% brine, lab. made) | 2.59 ± 0.98 a | 2.42 ± 0.03 a | <0.75 b | <0.75 b | N | 10 mg/kg | N | 5, 50 mg/kg |
| 5 | Anchovy, Brined-2 (15% brine), Turkey | NA | 319.05 ± 0.72 a | 320.00 ± 5.65 a | >250 a | P | 50, 100, 200 mg/kg | P | 50, 200 mg/kg |
| (>250) | |||||||||
| 6 | Anchovy, Brined-3, Turkey | 15.17 ± 0.40 a | 10.50 ± 0.18 b | 12.08 ± 1.25 b | 14.11 ± 0.18 a | P | 10 mg/kg | P | 10, 15 mg/kg |
| N | 25 mg/kg | N | 15, 25, 50 mg/kg | ||||||
| 7 | Anchovy, Brined-5, Turkey | 36.20 ± 2.56 a | 35.71 ± 0.86 a | 34.19 ± 2.79 a | 36.10 ± 1.50 a | P | 25 mg/kg | P | 10, 25 mg/kg |
| N | 50, 100 mg/kg | N | 50, 100 mg/kg | ||||||
| 8 | Anchovy, Brined-5 (20% brine), Turkey | NA | 72.94 ± 1.86 a | 83.30 ± 7.92 a | 73.38 ± 2.74 a | P | 50 mg/kg | P | 50 mg/kg |
| N | 100 mg/kg | N | 100 mg/kg | ||||||
| 9 | Anchovy, in herbal sauce, Italy | 11.10 ± 0.01 a | 10.33 ± 0.55 a | 10.42 ± 0.68 a | 11.60 ± 0.20 a | P | NA | P | 5, 10 mg/kg |
| N | NA | N | 15, 25 mg/kg | ||||||
| 10 | Anchovy, Dry-salted in oil, Croatia | 6.06 ± 0.63 a | 6.47 ± 0.31 a | 6.95 ± 0.91 a | 6.15 ± 0.20 a | N | 10, 15 mg/kg | P | 5 mg/kg |
| N | 5, 10, 15, 50 mg/kg | ||||||||
| 11 | Anchovy, Marinated-1 (in oil & vinegar), Italy | NA | 2.02 ± 0.05 a | 2.10 ± 0.36 a | 1.50 ± 0.20 a | N | 10 mg/kg | N | 5, 50 mg/kg |
| 12 | Anchovy, Marinated-2, Turkey | NA | 3.49 ± 0.17 a | 3.88 ± 0.50 a | 2.70 ± 0.26 b | N | 10 mg/kg | P | 5 mg/kg |
| N | 10, 50 mg/kg | ||||||||
| 13 | Anchovy, Paste, Italy | 3.06 ± 0.03 a | 3.82 ± 0.11 b | 2.90 ± 0.18 a | 2.90 ± 0.20 a | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 14 | Anchovy-Smoked & Marinated, Turkey | 2.36 ± 0.48 a | 1.85 ± 0.07 a | <0.75 c | <0.75 c | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 15 | A. Bonito, Lakerda-1, Turkey | 1.78 ± 0.06 a | <0.85 b | <0.75 c | <0.75 c | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 16 | A. Bonito, Lakerda-2, Turkey | 2.59 ± 0.50 a | 1.07 ± 0.06 b | 1.00 ± 0.34 b | <0.75 c | N | 10 mg/kg | N | 5, 10 mg/kg |
| 17 | A. Bonito, Lakerda-3, Turkey | 20.47 ± 0.45 a | 20.29 ± 0.70 a | 20.34 ± 1.34 a | 20.63 ± 1.10 a | P | 5, 10, 15 mg/kg | P | 15 mg/kg |
| N | 50, 200 mg/kg | N | 50, 200 mg/kg | ||||||
| 18 | A. Bonito, Lakerda-4, Turkey | 196.67 ± 0.58 a | 205.87 ± 3.15 a | 158.2 ± 6.36 b | 201.59 ± 12.18 a | P | 200 mg/kg | P | 50, 200 mg/kg |
| 19 | Herring, Dry salted-1, Turkey | 2.36 ± 0.58 a | 2.91 ± 0.11 a | 1.74 ± 0.98 ab | <0.75 c | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 20 | Herring, Dry salted-2, Turkey | 9.03 ± 0.20 a | 8.14 ± 0.46 a | 7.60 ± 0.16 a | 8.91 ± 0.20 a | P N | 10 mg/kg | P N | 5, 10 mg/kg |
| 15 mg/kg | 10, 15, 25 mg/kg | ||||||||
| 21 | Herring, Marinated-1, Romania | 1.59 ± 0.12 a | 1.28 ± 0.02 b | <0.75 c | <0.75 c | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 22 | Herring, Marinated-2, Turkey | 1.38 ± 0.54 a | <0.85 b | <0.75 c | <0.75 c | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 23 | Herring, Fermented-1, Denmark | 11.25 ± 0.49 a | 8.98 ± 0.36 b | 9.73 ± 1.39 ab | 11.60 ± 0.40 a | P N | 10, 15 mg/kg | P N | 10, 15 mg/kg |
| 25, 50 mg/kg | 15, 25, 50 mg/kg | ||||||||
| 24 | Herring, Fermented-2, Denmark | 12.55 ± 3.10 a | 9.31 ± 0.40 a | 9.42 ± 1.09 a | 10.70 ± 0.40 a | N | 25, 50 mg/kg | P N | 5, 10 mg/kg |
| 15, 25, 50 mg/kg | |||||||||
| 25 | Salmon, Smoked, Norway | 1.67 ± 0.09 a | 1.81 ± 0.06 a | <0.75 b | <0.75 c | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 26 | Salmon, Gravadlax-1, Scotland | 21.99 ± 1.97 a | 23.12 ± 1.23 a | 24.30 ± 4.38 a | 25.10 ± 1.80 ab | P N | 15 mg/kg | P N | 10, 15 mg/kg |
| 25, 50, 100 mg/kg | 25, 50, 100 mg/kg | ||||||||
| 27 | Salmon, Gravadlax-2, Scotland | 35.10 ± 5.80 a | 21.29 ± 4.00 b | 37.60 ± 2.83 a | 35.20 ± 2.43 a | P N | 10, 15 mg/kg | P N | 15, 25 mg/kg |
| 25, 50, 100 mg/kg | 50, 100 mg/kg | ||||||||
| 28 | Salmon paste, Germany | NA | 4.95 ± 0.07 a | 3.58 ± 0.13 b | 2.70 ± 0.10 b | N | 10 mg/kg | P N | 5 mg/kg |
| 10, 50 mg/kg | |||||||||
| 29 | Sardine, Canned, Slovenia | NA | <0.85 a | <0.75 a | <0.75 a | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 30 | Tuna, Paste, Denmark | 2.10 ± 0.40 a | <0.85 b | 1.30 ± 0.13 c | 1.60 ± 0.10 a | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 31 | Tuna, Smoked, Lithuania | 0.30 ± 0.02 a | <0.85 a | <0.75 a | <0.75 a | N | 10 mg/kg | N | 5, 10, 50 mg/kg |
| 32 | Tuna, Smoked, Europe | 2.60 ± 0.01 a | <0.85 b | <0.75 c | <0.75 c | N | 10 mg/kg | P | 5 mg/kg |
| N | 10, 50 mg/kg | ||||||||
| Biogenic Amines (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | Methods | TRP | PHE | PUT | CAD | TYR | SPD | SPM |
| Fresh Anchovy-1, Turkey | IC-MS/MS | 1.63 ± 0.07 a | 1.63 ± 0.01 a | 4.43 ± 0.34 a | 12.15 ± 0.49 a | 3.40 ± 0.09 a | 0.53 ± 0.10 a | 2.62 ± 0.75 a |
| HPLC | 8.90 ± 0.96 b | 0.84 ± 0.18 *2,b | 2.16 ± 0.15 b | 9.36 ± 0.59 b | 3.10 ± 0.04 a | 0.36 ± 0.03 *6,b | 1.69 ± 0.09 b | |
| Fresh Anchovy-2, Turkey | IC-MS/MS | 1.57 ± 0.04 a | 0.18 ± 0.03 *1,a | 3.21 ± 0.25 a | 5.38 ± 0.01 a | 1.44 ± 0.14 a | 0.73 ± 0.21 a | 2.33 ± 0.87 a |
| HPLC | 10.34 ± 0.11 b | 0.28 ± 0.04 *2,a | 1.68 ± 0.20 b | 5.52 ± 0.51 a | <0.90 b | 0.47 ± 0.04 *6,b | 1.59 ± 0.02 a | |
| Anchovy, Brined-1 (15% brine, lab. made) | IC-MS/MS | 2.67 ± 1.03 a | 0.19 ± 0.01 *1,a | 5.39 ± 1.84 a | 11.35 ± 0.92 a | 2.16 ± 0.06 a | 2.85 ± 0.21 a | 1.75 ± 0.35 a |
| HPLC | 39.18 ± 2.06 b | 2.40 ± 0.16 b | 0.32 ± 0.07 *3,b | 10.20 ± 0.31 a | 1.56 ± 0.17 b | 9.90 ± 0.34 b | 0.39 ± 0.06 *7,b | |
| Anchovy, Brined-2, Turkey | IC-MS/MS | 24.40 ± 0.70 a | 3.73 ± 0.03 a | 43.95 ± 1.90 a | 3.40 ± 0.88 a | 46.02 ± 0.90 a | 5.46 ± 3.17 a | 5.83 ± 1.70 a |
| HPLC | 36.85 ± 2.90 b | 2.25 ± 1.42 a | 40.81 ± 0.59 a | 4.57 ± 0.38 a | 39.34 ± 0.74 b | 9.48 ± 0.57 b | 0.79 ± 0.05 b | |
| Anchovy, Brined-3, Turkey | IC-MS/MS | 32.23 ± 0.93 a | 4.67 ± 0.21 a | 57.57 ± 1.19 a | 3.05 ± 0.77 a | 53.98 ± 0.69 a | 7.93 ± 0.69 a | 2.99 ± 0.84 a |
| HPLC | 22.84 ± 0.83 b | 5.51 ± 0.60 a | 55.36 ± 1.35 a | 3.84 ± 0.36 a | 60.90 ± 0.20 b | 8.31 ± 0.33 a | 0.95 ± 0.09 b | |
| Anchovy, in herbal sauce, Italy | IC-MS/MS | 1.69 ± 0.06 a | 2.17 ± 0.07 a | 3.57 ± 0.09 a | 5.12 ± 0.24 a | 2.40 ± 0.07 a | 4.09 ± 0.71 a | 3.26 ± 0.09 a |
| HPLC | 10.32 ± 0.18 b | 2.81 ± 0.14 b | 1.36 ± 0.16 b | 9.15 ± 1.12 b | 2.30 ± 0.20 a | 5.21 ± 1.10 a | 1.35 ± 0.17 b | |
| Anchovy, Dry-salted in oil, Croatia | IC-MS/MS | 1.55 ± 0.01 a | 2.08 ± 0.10 a | 5.46 ± 0.46 a | 9.49 ± 0.62 a | 3.99 ± 0.31 a | 9.48 ± 0.09 a | 2.87 ± 0.75 a |
| HPLC | 6.58 ± 0.16 b | 4.60 ± 0.08 b | 2.72 ± 0.20 b | 9.98 ± 0.34 a | 4.87 ± 0.30 b | 9.28 ± 0.14 a | 1.54 ± 0.13 b | |
| Anchovy, Paste, Italy | IC-MS/MS | 5.56 ± 0.34 a | 2.21 ± 0.09 a | 4.97 ± 0.20 a | 12.50 ± 0.26 a | 6.18 ± 0.24 a | 11.81 ± 1.88 a | 3.56 ± 0.23 a |
| HPLC | 5.42 ± 0.30 a | 5.32 ± 0.44 b | 4.12 ± 0.12 b | 24.20 ± 0.88 b | 8.98 ± 0.34 b | 8.46 ± 0.68 b | 2.54 ± 0.48 b | |
| Anchovy-Smoked & Marinated, Turkey | IC-MS/MS | 1.62 ± 0.08 a | 1.64 ± 0.02 a | 3.30 ± 0.48 a | 3.19 ± 0.33 a | 1.33 ± 0.05 a | 8.98 ± 2.02 a | 2.82 ± 0.71 a |
| HPLC | 5.34 ± 0.19 b | 10.31 ± 0.64 b | 2.74 ± 0.15 a | 6.07 ± 0.93 b | 0.66 ± 0.11 *5,b | 8.34 ± 0.22 a | 0.63 ± 0.67 *7,b | |
| A. Bonito, Lakerda-1, Turkey | IC-MS/MS | 5.33 ± 0.40 a | 3.20 ± 0.32 a | 30.20 ± 0.98 a | 53.87 ± 6.29 a | 46.73 ± 1.97 a | 21.65 ± 2.28 a | 18.45 ± 3.14 a |
| HPLC | 5.12 ± 0.28 a | 1.08 ± 0.14 *1,b | 31.05 ± 1.14 a | 52.38 ± 3.54 a | 47.17 ± 2.46 a | 23.22 ± 1.52 a | <0.71 b | |
| A. Bonito, Lakerda-2, Turkey | IC-MS/MS | 0.19 ± 0.01 *1,a | 1.63 ± 0.03 a | 2.80 ± 0.29 a | 2.59 ± 0.01 a | 1.38 ± 0.06 a | 3.99 ± 0.16 a | 2.94 ± 0.17 a |
| HPLC | 4.88 ± 0.26 b | 15.16 ± 0.34 b | <0.56 b | 1.53 ± 0.20 b | 3.02 ± 0.18 b | 54.16 ± 3.12 b | 3.30 ± 0.22 a | |
| A. Bonito, Lakerda-3, Turkey | IC-MS/MS | 1.66 ± 0.07 a | 1.07 ± 0.84 a | 36.47 ± 0.93 a | 155.00 ± 1.41 a | 86.40 ± 3.89 a | 4.14 ± 1.17 a | 2.60 ± 0.95 a |
| HPLC | 5.11 ± 0.04 b | 0.07 ± 0.07 *2,b | 28.99 ± 0.79 b | 111.59 ± 2.37 b | 87.38 ± 2.48 a | 22.02 ± 0.69 b | 0.96 ± 0.12 b | |
| A. Bonito, Lakerda-4, Turkey | IC-MS/MS | 2.11 ± 0.77 a | 0.18 ± 0.02 a | 3.39 ± 1.31 a | 9.60 ± 0.55 a | 2.03 ± 0.32 a | 3.15 ± 0.45 a | 3.04 ± 0.57 a |
| HPLC | 5.68 ± 0.09 b | 17.49 ± 0.99 b | 0.43 ± 0.11 *3,b | 8.46 ± 0.19 a | 0.62 ± 0.18 *5,b | 15.01 ± 0.87 b | 0.99 ± 0.13 b | |
| Herring, Dry salted-1, Turkey | IC-MS/MS | 0.18 ± 0.03 *1,a | 0.18 ± 0.03 *1,a | 5.53 ± 0.20 a | 18.37 ± 0.50 a | 1.52 ± 0.04 a | 9.61 ± 0.32 a | 2.78 ± 0.71 a |
| HPLC | 6.73 ± 0.23 b | 4.11 ± 0.35 b | 2.98 ± 0.30 b | 15.95 ± 1.12 a | 0.45 ± 0.16 *5,b | 11.10 ± 0.25 b | 1.33 ± 0.15 b | |
| Herring, Marinated-1, Romania | IC-MS/MS | 27.13 ± 1.59 a | 1.85 ± 0.10 a | 2.66 ± 0.09 a | 3.61 ± 0.06 a | 27.28 ± 0.76 a | 2.67 ± 0.15 a | 2.50 ± 0.98 a |
| HPLC | 29.06 ± 1.26 a | 2.84 ± 0.16 b | 0.32 ± 0.11 *3,b | 4.38 ± 0.68 b | 41.56 ± 3.12 b | 8.44 ± 0.52 b | 0.89 ± 0.10 *7,b | |
| Herring, Marinated-2, Turkey | IC-MS/MS | 9.03 ± 0.35 a | 4.87 ± 0.23 a | 5.50 ± 0.70 a | 16.60 ± 1.56 a | 3.20 ± 0.08 a | 10.04 ± 0.66 a | 3.46 ± 0.64 a |
| HPLC | 36.06 ± 1.52 b | 5.05 ± 0.10 a | 0.99 ± 0.33 b | 1.75 ± 0.10 b | 1.90 ± 0.16 b | 10.33 ± 0.78 a | 1.75 ± 0.02 b | |
| Herring, Fermented-1, Denmark | IC-MS/MS | 6.77 ± 0.61 a | 2.97 ± 0.11 a | 18.55 ± 0.64 a | 44.37 ± 1.98 a | 22.23 ± 0.90 a | 15.50 ± 1.13 a | 3.89 ± 0.61 a |
| HPLC | 9.12 ± 0.90 b | 2.72 ± 0.40 a | 16.88 ± 1.32 a | 45.08 ± 2.18 a | 30.42 ± 2.37 b | 6.89 ± 0.78 b | 1.78 ± 0.21 b | |
| Herring, Fermented-2, Denmark | IC-MS/MS | 1.67 ± 0.02 a | 2.15 ± 0.02 a | 21.53 ± 0.85 a | 46.20 ± 3.14 a | 20.33 ± 0.12 a | 14.07 ± 0.51 a | 2.13 ± 0.35 a |
| HPLC | 30.08 ± 3.12 b | 2.56 ± 0.30 a | 22.36 ± 2.08 a | 47.32 ± 2.08 a | 26.12 ± 2.08 b | 6.12 ± 0.42 a | 1.72 ± 0.30 b | |
| Salmon, Smoked, Norway | IC-MS/MS | 12.53 ± 0.84 a | 5.43 ± 0.19 a | 75.60 ± 0.71 a | 118.15 ± 1.63 a | 140.25 ± 2.99 a | 3.00 ± 0.19 a | 3.88 ± 0.69 a |
| HPLC | 17.88 ± 1.42 b | 26.18 ± 2.08 b | 148.08 ± 5.10 b | 121.18 ± 2.68 a | 166.38 ± 5.24 b | 7.44 ± 0.72 b | 4.28 ± 0.54 a | |
| Salmon, Gravadlax, Scotland | IC-MS/MS | 0.66 ± 0.80 a | 2.64 ± 0.71 a | 9.71 ± 0.70 a | 29.59 ± 0.55 a | 59.70 ± 0.71 a | 6.03 ± 0.97 a | 3.13 ± 0.10 a |
| HPLC | 5.38 ± 0.22 b | 5.92 ± 0.48 b | 10.48 ± 1.02 a | 33.88 ± 3.87 a | 75.12 ± 3.06 b | 6.98 ± 0.52 a | 8.03 ± 0.60 b | |
| Tuna, Paste, Denmark, | IC-MS/MS | 0.18 ± 0.03 *1,a | 1.70 ± 0.04 a | 5.53 ± 0.75 a | 5.89 ± 0.62 a | 3.59 ± 0.11 a | 8.02 ± 0.25 a | 4.20 ± 0.73 a |
| HPLC | 6.62 ± 0.72 b | 9.44 ± 0.58 b | 3.74 ± 0.22 b | 3.86 ± 0.52 b | 3.72 ± 0.40 a | 43.22 ± 3.34 b | 4.46 ± 0.72 a | |
| Tuna, Smoked, Lithuania | IC-MS/MS | 2.33 ± 0.55 a | 1.64 ± 0.01 a | 3.58 ± 0.35 a | 4.39 ± 0.36 a | 4.69 ± 0.08 a | 6.62 ± 0.52 a | 5.86 ± 0.21 a |
| HPLC | 6.54 ± 0.34 b | 118.36 ± 6.12 b | 1.32 ± 0.26 b | <0.87 *4, b | 19.06 ± 2.42 b | 53.12 ± 4.17 b | 5.90 ± 0.68 a | |
| Tuna, Smoked, Europe | IC-MS/MS | 0.19 ± 0.01 *1,a | 0.15 ± 0.00 *1,a | 3.47 ± 0.57 a | 0.80 ± 0.07 a | 1.49 ± 0.12 a | 2.00 ± 0.00 a | 31.20 ± 0.57 a |
| HPLC | 3.94 ± 0.18 b | <1.50 a | <0.56 b | <0.66 b | <0.87 b | 1.22 ± 0.24 b | 0.96 ± 0.20 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kočar, D.; Köse, S.; Koral, S.; Tufan, B.; Ščavničar, A.; Pompe, M. Analysis of Biogenic Amines Using Immunoassays, HPLC, and a Newly Developed IC-MS/MS Technique in Fish Products—A Comparative Study. Molecules 2021, 26, 6156. https://doi.org/10.3390/molecules26206156
Kočar D, Köse S, Koral S, Tufan B, Ščavničar A, Pompe M. Analysis of Biogenic Amines Using Immunoassays, HPLC, and a Newly Developed IC-MS/MS Technique in Fish Products—A Comparative Study. Molecules. 2021; 26(20):6156. https://doi.org/10.3390/molecules26206156
Chicago/Turabian StyleKočar, Drago, Sevim Köse, Serkan Koral, Bekir Tufan, Andrej Ščavničar, and Matevž Pompe. 2021. "Analysis of Biogenic Amines Using Immunoassays, HPLC, and a Newly Developed IC-MS/MS Technique in Fish Products—A Comparative Study" Molecules 26, no. 20: 6156. https://doi.org/10.3390/molecules26206156