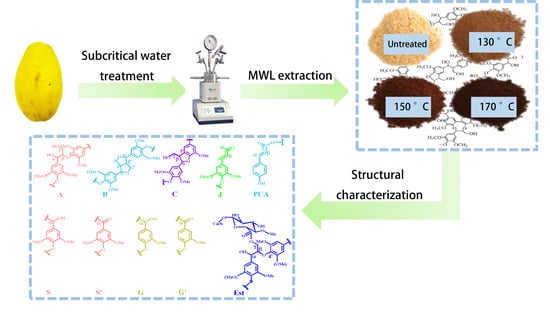
Structural Changes in Milled Wood Lignin (MWL) of Chinese Quince (Chaenomeles sinensis) Fruit Subjected to Subcritical Water Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical and Morphological Changes of Chinese Quince Fruit Residues during Subcritical Water Treatment
2.2. Yield and Carbohydrate Components of MWLs
2.3. FT-IR Analysis
2.4. Molecular Weight Distributions of MWLs
2.5. Thermal Analysis
2.6. Py-GC/MS Analysis
2.7. NMR Analysis
2.7.1. 2D-HSQC-NMR
2.7.2. 31P-NMR Analysis
3. Materials and Methods
3.1. Materials
3.2. Subcritical Water Treatment
3.3. Scanning Electron Microscopy (SEM)
3.4. Preparation of MWL Fractions
3.5. Structural Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yin, H.S.; Liu, H.M.; Liu, Y.L. Structural characterization of lignin in fruits and stalks of Chinese quince. Molecules 2017, 22, 890. [Google Scholar]
- Sawai-Kuroda, R.; Kikuchi, S.; Shimizu, Y.K.; Sasaki, Y.; Kuroda, K.; Tanaka, T.; Yamamoto, T.; Sakurai, K.; Shimizu, K. A polyphenol-rich extract from Chaenomeles sinensis (Chinese quince) inhibits influenza A virus infection by preventing primary transcription in vitro. J. Ethnopharmacol. 2013, 146, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Hamauzu, Y.; Irie, M.; Kondo, M.; Fujita, T. Antiulcerative properties of crude polyphenols and juice of apple, and Chinese quince extracts. Food Chem. 2008, 108, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Zhang, Z.G.; Liu, H.M.; Qin, G.Y.; Wang, X.D. Acetic acid lignins from Chinese quince fruit (Chaenomeles sinensis): Effect of pretreatment on their structural features and antioxidant activities. RSC Adv. 2018, 8, 24923–24931. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yuan, T.Q.; Sun, R.C. Structural elucidation of whole lignin in cell walls of triploid of Populus tomentosa Carr. ACS Sustain. Chem. Eng. 2016, 4, 1006–1015. [Google Scholar] [CrossRef]
- Zhao, B.C.; Chen, B.Y.; Yang, S.; Yuan, T.Q.; Charlton, A.; Sun, R.C. Structural variation of lignin and lignin-carbohydrate complex in Eucalyptus grandis × E. urophylla during its growth process. ACS Sustain. Chem. Eng. 2016, 5, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Zhang, L.L.; Yu, J.; Lu, Y.Z.; Jiang, B.; Fan, Y.M.; Wang, Z.G. High-purity lignin isolated from poplar wood meal through dissolving treatment with deep eutectic solvents. Soc. Open Sci. 2019, 6, 181757. [Google Scholar] [CrossRef] [Green Version]
- Nylander, F.; Sunnera, H.; Olsson, L.; Christakopoulos, P.; Westman, G. Synthesis and enzymatic hydrolysis of a diaryl benzyl ester model of a lignin-carbohydrate complex (LCC). Holzforschung 2016, 70, 385–391. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, L.; Wang, B.; Xu, F.; Sun, R.C. Microwave-enhanced extraction of lignin from birch in formic acid: Structural characterization and antioxidant activity study. Process Biochem. 2012, 47, 1799–1806. [Google Scholar] [CrossRef]
- Wen, J.L.; Yuan, T.Q.; Sun, S.L.; Xu, F.; Sun, R.C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Yao, K.; Wu, Q.F.; An, R.; Meng, W.; Ding, M.Z.; Li, B.Z.; Yuan, Y.J. Hydrothermal pretreatment for deconstruction of plant cell wall: Part I. Effect on lignin-carbohydrate complex. AIChE J. 2018, 64, 1938–1953. [Google Scholar] [CrossRef]
- Liu, H.M.; Li, Y.R.; Wu, M.; Yin, H.S.; Wang, X.D. Two-step isolation of hemicelluloses from Chinese quince fruit: Effect of hydrothermal treatment on structural features. Ind. Crops Prod. 2018, 111, 615–624. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Characterization of MWLs from Tamarix ramosissima isolated before and after hydrothermal treatment by spectroscopical and wet chemical methods. Holzforschung 2012, 66, 295–302. [Google Scholar] [CrossRef]
- Kaparaju, P.; Felby, C. Characterization of lignin during oxidative and hydrothermal pre-treatment processes of wheat straw and corn stover. Bioresour. Technol. 2010, 101, 3175–3181. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, K.; Song, X.L.; Xu, F.; Sun, R.C. Enhanced enzymatic hydrolysis of triploid poplar following stepwise acidic pretreatment and alkaline fractionation. Process Biochem. 2012, 47, 619–625. [Google Scholar] [CrossRef]
- Wang, C.Z.; Li, H.Y.; Li, M.F.; Bian, J.; Sun, R.C. Revealing the structure and distribution changes of Eucalyptus lignin during the hydrothermal and alkaline pretreatments. Sci. Rep. 2017, 7, 593. [Google Scholar] [CrossRef]
- Xiao, X.; Bian, J.; Li, M.F.; Xu, H.; Xiao, B.; Sun, R.C. Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment. Bioresour. Technol. 2014, 159, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.Q.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Niu, W.S.; Zhang, A.D.; Yi, W.M. Enhanced anaerobic digestion of corn stover by thermo-chemical pretreatment. Int. J. Agric. Biol. Eng. 2015, 8, 84–90. [Google Scholar]
- Li, M.F.; Chen, C.Z.; Sun, R.C. Effect of pretreatment severity on the enzymatic hydrolysis of bamboo in hydrothermal deconstruction. Cellulose 2014, 21, 4105–4117. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.M.; Lv, T.T.; Wang, X.D. Structure, rheological, thermal and antioxidant properties of cell wall polysaccharides from Chinese quince fruits. Int. J. Biol. Macromol. 2020, 147, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Xu, F.; Sun, R.C. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Wang, R.C.; Kobayashi, T.; Adachi, S. Degradation kinetics of D-galacturonic acid and sodium D-galacturonate in subcritical water. J. Appl. Glycosci. 2009, 56, 181–184. [Google Scholar] [CrossRef]
- DeMartini, J.D.; Pattathil, S.; Avci, U.; Szekalski, K.; Mazumder, K.; Hahn, M.G.; Wyman, C.E. Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy Environ. Sci. 2011, 4, 4332–4339. [Google Scholar] [CrossRef]
- Ma, M.G.; Jia, N.; Zhu, J.F.; Li, S.M.; Peng, F.; Sun, R.C. Isolation and characterization of hemicelluloses extracted by hydrothermal pretreatment. Bioresour. Technol. 2012, 114, 677–683. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renewable Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.Y.; Zhang, P.Y.; Li, F.; Jin, S.G.; Wang, S.Q.; Zhou, S.Q. A review of lignocellulose change during hydrothermal pretreatment for bioenergy production. Curr. Org. Chem. 2016, 20, 2799–2809. [Google Scholar] [CrossRef]
- Moghaddam, L.; Rencoret, J.; Maliger, V.R.; Rackemann, D.W.; Harrison, M.D.; Gutierrez, A.; del Río, J.C.; Doherty, W.O.S. Structural characteristics of bagasse furfural residue and its lignin component. An NMR, Py-GC/MS, and FTIR Study. ACS Sustain. Chem. Eng. 2017, 5, 4846–4855. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C.; Jones, G.L. Structural features and antioxidant activities of degraded lignins from steam exploded bamboo stem. Ind. Crops Prod. 2014, 56, 128–136. [Google Scholar] [CrossRef]
- Yan, T.T.; Xu, Y.Q.; Yu, C.W. The isolation and characterization of lignin of kenaf fiber. J. Appl. Polym. Sci. 2009, 114, 1896–1901. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Zhang, L.G.; Hou, Q.X. Characterization and comparison of lignin derived from corncob residues to better understand its potential applications. Int. J. Biol. Macromol. 2019, 134, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Qin, Z.; Ma, Y.X.; Liu, H.M.; Qin, G.Y.; Wang, X.D. Structural elucidation of lignin-carbohydrate complexes (LCCs) from Chinese quince (Chaenomeles sinensis) fruit. Int. J. Biol. Macromol. 2018, 116, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Wu, J.Q.; Peng, W.X.; Xiao, W.F.; Wu, J.G.; Zhuo, J.Y.; Yuan, T.Q.; Sun, R.C. Effect of lignin on bamboo biomass self-bonding during hot-pressing: Lignin structure and characterization. BioResources 2015, 10, 6769–6782. [Google Scholar] [CrossRef] [Green Version]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Mulat, D.G.; Dibdiakova, J.; Horn, S.J. Microbial biogas production from hydrolysis lignin: Insight into lignin structural changes. Biotechnol. Biofuels 2018, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.J.; Xiao, L.P.; Deng, J.; Xu, F.; Sun, R.C. Physicochemical characterization of lignin fractions sequentially isolated from bamboo (Dendrocalamus brandisii) with hot water and alkaline ethanol solution. J. Appl. Polym. Sci. 2012, 125, 3290–3301. [Google Scholar] [CrossRef]
- Sun, S.N.; Li, H.Y.; Cao, X.F.; Xu, F.; Sun, R.C. Structural variation of eucalyptus lignin in a combination of hydrothermal and alkali treatments. Bioresour. Technol. 2015, 176, 296–299. [Google Scholar] [CrossRef]
- Rencoret, J.; Kim, H.; Evaristo, A.B.; Gutierrez, A.; Ralph, J.; del Rio, J.C. Variability in lignin composition and structure in cell walls of different parts of macaúba (Acrocomia aculeata) palm fruit. J. Agric. Food Chem. 2018, 66, 138–153. [Google Scholar] [CrossRef] [Green Version]
- Du, X.Y.; Gellerstedt, G.; Li, J.B. Universal fractionation of lignin-carbohydrate complexes (LCCs) from lignocellulosic biomass: An example using spruce wood. Plant J. 2013, 74, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Xue, Y.; Sharma, A.; Bai, X.L. Lignin valorization through thermochemical conversion: Comparison of hardwood, softwood and herbaceous lignin. ACS Sustain. Chem. Eng. 2016, 4, 6608–6617. [Google Scholar] [CrossRef]
- del Rio, J.C.; Prinsen, P.; Rencoret, J.; Nieto, L.; Jimenez-Barbero, J.; Ralph, J.; Martinez, A.T.; Gutierrez, A. Structural characterization of the lignin in the cortex and pith of elephant grass (Pennisetum purpureum) stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Characterization of lignins isolated with alkaline ethanol from the hydrothermal pretreated Tamarix ramosissima. BioEnergy Res. 2012, 6, 519–532. [Google Scholar] [CrossRef]
- Zhao, B.C.; Xu, J.D.; Chen, B.Y.; Cao, X.F.; Yuan, T.Q.; Wang, S.F.; Charlton, A.; Sun, R.C. Selective precipitation and characterization of lignin-carbohydrate complexes (LCCs) from Eucalyptus. Planta 2018, 247, 1077–1087. [Google Scholar] [CrossRef]
- Samuel, R.; Pu, Y.Q.; Raman, B.; Ragauskas, A.J. Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 62–74. [Google Scholar] [CrossRef]
- Dou, J.; Kim, H.; Li, Y.D.; Padmakshan, D.; Yue, F.X.; Ralph, J.; Vuorinen, T. Structural characterization of lignins from willow bark and wood. J. Agric. Food Chem. 2018, 66, 7294–7300. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C. Structural elucidation of sorghum lignins from an integrated biorefinery process based on hydrothermal and alkaline treatments. J. Agric. Food Chem. 2014, 62, 8120–8128. [Google Scholar] [CrossRef]
- Huang, C.X.; He, J.; Du, L.T.; Min, D.Y.; Yong, Q. Structural characterization of the lignins from the green and yellow bamboo of bamboo culm (Phyllostachys pubescens). J. Wood Chem. Technol. 2015, 36, 157–172. [Google Scholar] [CrossRef]
- Brosse, N.; Hage, R.E.; Chaouch, M.; Pétrissans, M.; Dumarçay, S.; Gérardin, P. Investigation of the chemical modifications of beech wood lignin during heat treatment. Polym. Degrad. Stab. 2010, 95, 1721–1726. [Google Scholar] [CrossRef]
- Cheng, X.C.; Guo, X.R.; Qin, Z.; Wang, X.D.; Liu, H.M.; Liu, Y.L. Structural features and antioxidant activities of Chinese quince (Chaenomeles sinensis) fruits lignin during auto-catalyzed ethanol organosolv pretreatment. Int. J. Biol. Macromol. 2020, 164, 4348–4358. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liu, H.M.; Ma, Y.X.; Wang, X.D. Characterization of fragrant oil extracted from pepper seed during subcritical propane extraction. LWT-Food Sci. Technol. 2019, 110, 110–116. [Google Scholar] [CrossRef]
- Björkman, A. Studies on finely divided wood. Part 1. Extraction of lignin with neutral solvents. Sven. Papperstidn 1956, 59, 477–485. [Google Scholar]
- Kang, S.M.; Xiao, L.P.; Meng, L.Y.; Zhang, X.M.; Sun, R.C. Isolation and structural characterization of lignin from cotton stalk treated in an ammonia hydrothermal system. Int. J. Mol. Sci. 2012, 13, 15209–15226. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Ultrasound-enhanced extraction of lignin from bamboo (Neosinocalamus affinis): Characterization of the ethanol-soluble fractions. Ultrason. Sonochem. 2012, 19, 243–249. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Tompsettb, G.A.; Guerrab, P.; Timkob, M.T.; Rostagnoc, M.A.; Martíneza, J.; Forster-Carneiro, T. Sugars and char formation on subcritical water hydrolysis of sugarcane straw. Bioresour. Technol. 2017, 243, 1069–1077. [Google Scholar] [CrossRef]
- Yang, L.N.; Wang, D.M.; Zhou, D.; Zhang, Y.W.; Yang, T.T. Isolation and further structural characterization of lignins from the valonea of Quercus variabilis. Int. J. Biol. Macromol. 2017, 97, 164–172. [Google Scholar] [CrossRef]




| Sample a | Yield b (%) | Sugar Content (%) | Carbohydrate Composition Content (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fuc c | Ara c | Gal c | Glc c | Xyl c | Gal-A c | Glc-A c | |||
| UL | 0.04 | 0.62 | 0.06 | 1.05 | 6.12 | 20.60 | 1.08 | 62.63 | 8.47 |
| L130 | 0.39 | 0.16 | 0.68 | 3.88 | 10.70 | 23.08 | 11.42 | 47.92 | 2.34 |
| L150 | 0.54 | 0.11 | 0.83 | 10.22 | 7.28 | 21.14 | 8.21 | 50.41 | 1.91 |
| L170 | 3.33 | 0.25 | 1.14 | 19.70 | 3.63 | 8.98 | 44.61 | 21.61 | 0.33 |
| Sample | Mw | Mn | Mp | Mw/Mn |
|---|---|---|---|---|
| UL | 16,543 | 5276 | 16,190 | 3.14 |
| L130 | 12,204 | 6086 | 7801 | 2.01 |
| L150 | 10,516 | 5444 | 8389 | 1.93 |
| L170 | 13,524 | 6648 | 10,807 | 2.03 |
| Lignin Interunit Linkages | Percentage (%) | |
|---|---|---|
| UL | L170 | |
| β-O-4′ aryl ethers (A) | 74.11 | 72.26 |
| β- β′ (resinols) (B) | 19.78 | 21.08 |
| β-5′ (phenylcoumarans) (C) | 6.11 | 6.66 |
| Syringyl units (S2,6) | 69.04 | 82.83 |
| Guaiacyl units (G2) | 23.34 | 12.29 |
| p-Hydroxyphenyl units (H2,6) | 7.63 | 4.88 |
| S/G ratio | 1.48 | 3.37 |
| Samples | Aliphatic OH | Syringyl OH | Guaiacyl OH | p-Hydroxy Phenyl | Carboxylic Group |
|---|---|---|---|---|---|
| UL | 1.30 | 0.04 | 0.36 | 0.05 | 0.02 |
| L130 | 1.33 | 0.04 | 0.30 | 0.05 | 0.06 |
| L150 | 0.92 | 0.04 | 0.24 | 0.06 | 0.06 |
| L170 | 1.30 | 0.23 | 1.07 | 0.14 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-Y.; Qin, Z.; Liu, H.-M.; Wang, X.-D.; Gao, J.-H.; Qin, G.-Y. Structural Changes in Milled Wood Lignin (MWL) of Chinese Quince (Chaenomeles sinensis) Fruit Subjected to Subcritical Water Treatment. Molecules 2021, 26, 398. https://doi.org/10.3390/molecules26020398
Wang W-Y, Qin Z, Liu H-M, Wang X-D, Gao J-H, Qin G-Y. Structural Changes in Milled Wood Lignin (MWL) of Chinese Quince (Chaenomeles sinensis) Fruit Subjected to Subcritical Water Treatment. Molecules. 2021; 26(2):398. https://doi.org/10.3390/molecules26020398
Chicago/Turabian StyleWang, Wen-Yue, Zhao Qin, Hua-Min Liu, Xue-De Wang, Jing-Hao Gao, and Guang-Yong Qin. 2021. "Structural Changes in Milled Wood Lignin (MWL) of Chinese Quince (Chaenomeles sinensis) Fruit Subjected to Subcritical Water Treatment" Molecules 26, no. 2: 398. https://doi.org/10.3390/molecules26020398