Ethyl Lauroyl Arginate, an Inherently Multicomponent Surfactant System
Abstract
:1. Introduction
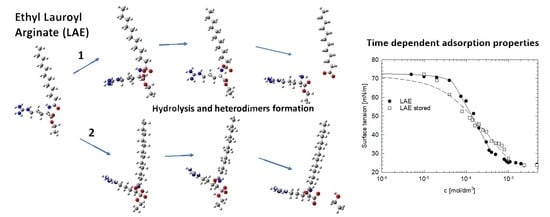
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Surface Tension and Elasticity
3.3. Quantum Chemical DFT Computations
3.4. Molecular Dynamics Calculations
3.5. Infrared Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tripathy, D.B.; Mishra, A.; Clark, J.; Farmer, T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. C. R. Chim. 2018, 21, 112–130. [Google Scholar] [CrossRef]
- Asker, D.; Weiss, J.; McClements, D.J. Analysis of the interactions of a cationic surfactant (Lauric arginate) with an anionic biopolymer (Pectin): Isothermal titration calorimetry, light scattering, and microelectrophoresis. Langmuir 2009, 25, 116–122. [Google Scholar] [CrossRef]
- Nerin, C.; Becerril, R.; Manso, S.; Silva, F. Ethyl Lauroyl Arginate (LAE): Antimicrobial Activity and Applications in Food Systems. In Antimicrobial Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 305–312. ISBN 9780128007235. [Google Scholar]
- Ruckman, S.A.; Rocabayera, X.; Borzelleca, J.F.; Sandusky, C.B. Toxicological and metabolic investigations of the safety of N-α-Lauroyl-l-arginine ethyl ester monohydrochloride (LAE). Food Chem. Toxicol. 2004, 42, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Lozano, N.; Pérez, L.; Pons, R.; Luque-Ortega, J.R.; Fernández-Reyes, M.; Rivas, L.; Pinazo, A. Interaction studies of diacyl glycerol arginine-based surfactants with DPPC and DMPC monolayers, relation with antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2008, 319, 196–203. [Google Scholar] [CrossRef]
- Rodríguez, E.; Seguer, J.; Rocabayera, X.; Manresa, A. Cellular effects of monohydrochloride of l-arginine, Nα-lauroyl ethylester (LAE) on exposure to Salmonella typhimurium and Staphylococcus aureus. J. Appl. Microbiol. 2004, 96, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Properties and potential food applications of lauric arginate as a cationic antimicrobial. Int. J. Food Microbiol. 2020, 315, 108417. [Google Scholar] [CrossRef] [PubMed]
- Bonnaud, M.; Weiss, J.; McClements, D.J. Interaction of a food-grade cationic surfactant (Lauric Arginate) with food-grade biopolymers (pectin, carrageenan, xanthan, alginate, dextran, and chitosan). J. Agric. Food Chem. 2010, 58, 9770–9777. [Google Scholar] [CrossRef]
- Asker, D.; Weiss, J.; McClements, D.J. Formation and stabilisation of antimicrobial delivery systems based on electrostatic complexes of cationic-non-ionic mixed micelles and anionic polysaccharides. J. Agric. Food Chem. 2011, 59, 1041–1049. [Google Scholar] [CrossRef]
- Loeffler, M.; Mcclements, D.J.; Mclandsborough, L.; Terjung, N.; Chang, Y.; Weiss, J. Electrostatic interactions of cationic lauric arginate with anionic polysaccharides affect antimicrobial activity against spoilage yeasts. J. Appl. Microbiol. 2014, 117, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Czakaj, A.; Kannan, A.; Wisniewska, A.; Grzes, G.; Krzan, M.; Warszynski, P.; Fuller, G.G. Viscoelastic interfaces comprising of cellulose nanocrystals and lauroyl ethyl arginate for enhanced foam stability. Soft Matter 2020, 16, 3981–3990. [Google Scholar] [CrossRef]
- Bai, L.; Xiang, W.; Huan, S.; Rojas, O.J. Formulation and Stabilization of Concentrated Edible Oil-in-Water Emulsions Based on Electrostatic Complexes of a Food-Grade Cationic Surfactant (Ethyl Lauroyl Arginate) and Cellulose Nanocrystals. Biomacromolecules 2018, 19, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Chi, K.; Catchmark, J.M. Crystalline nanocellulose/lauric arginate complexes. Carbohydr. Polym. 2017, 175, 320–329. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; Mennes, W.; et al. Safety of ethyl lauroyl arginate (E 243) as a food additive in the light of the new information provided and the proposed extension of use. EFSA J. 2019, 17, 5621. [Google Scholar]
- Infante, R.; Domínguez, J.G.; Erra, P.; Julia, R.; Prats, M. Surface active molecules: Preparation and properties of long chain nα-acyl-l-α-amino-ω-guanidine alkyl acid derivatives. Int. J. Cosmet. Sci. 1984, 6, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Whitehouse, B. Ethyl Lauroyl Arginate. Chemical and Technical Assessment; FAO: Rome, Italy, 2008. [Google Scholar]
- Singare, P.U.; Mhatre, J.D. Cationic Surfactants from Arginine: Synthesis and Physicochemical Properties. Am. J. Chem. 2012, 2, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, R.; Baba, K.; Saito, T.; Yoshimura, I. Surfactants Derived from Amino Acids. III. J. Jpn. Oil Chem. Soc. 1976, 25, 404–408. [Google Scholar] [CrossRef]
- Takehara, M. Properties and applications of amino acid based surfactants. Colloids Surf. 1989, 38, 149–167. [Google Scholar] [CrossRef]
- Para, G.; Jarek, E.; Warszynski, P. The Hofmeister series effect in adsorption of cationic surfactants—Theoretical description and experimental results. Adv. Colloid Interface Sci. 2006, 122, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk, D.; Ha̧c, K.; Wydro, P.S. Surface properties of the binary mixed systems of alkylpyridinium halides and sodium alkylsulfonates. Colloids Surf. A Physicochem. Eng. Asp. 2003, 220, 55–60. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Barzyk, W.; Hirte, R.; Rudert, R. Adsorption properties of soluble, surface-chemically pure n-alkanoic acids at the air/water interface and the relationship to insoluble monolayer and crystal structure properties. Langmuir 2003, 19, 6140–6150. [Google Scholar] [CrossRef]
- Jarek, E.; Wydro, P.; Warszyński, P.; Paluch, M. Surface properties of mixtures of surface-active sugar derivatives with ionic surfactants: Theoretical and experimental investigations. J. Colloid Interface Sci. 2006, 293, 194–202. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Haage, K.; Miller, R. On the adsorption properties of surface-chemically pure aqueous solutions of n-alkyl-dimethyl and n-alkyl-diethyl phosphine oxides. Colloids Surf. 1987, 22, 207–214. [Google Scholar] [CrossRef]
- Para, G.; Łuczyński, J.; Palus, J.; Jarek, E.; Wilk, K.A.K.A.; Warszyński, P. Hydrolysis driven surface activity of esterquat surfactants. J. Colloid Interface Sci. 2016, 465, 174–182. [Google Scholar] [CrossRef]
- Para, G.; Hamerska-Dudra, A.; Wilk, K.A.; Warszyński, P. Surface activity of cationic surfactants, influence of molecular structure. Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 215–221. [Google Scholar] [CrossRef]
- Para, G.; Hamerska-Dudra, A.; Wilk, K.A.; Warszyński, P. Mechanism of cationic surfactant adsorption—Effect of molecular structure and multiple charge. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 67–72. [Google Scholar] [CrossRef]
- Aznar, M.; Gómez-Estaca, J.; Vélez, D.; Devesa, V.; Nerín, C. Migrants determination and bioaccessibility study of ethyl lauroyl arginate (LAE) from a LAE based antimicrobial food packaging material. Food Chem. Toxicol. 2013, 56, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Application A1015 Ethyl Lauroyl Arginate as a Food Additive Approval Report. Available online: https://www.foodstandards.gov.au/code/applications/documents/A1015%20Lauric%20Arginate%20AppR%20FINAL1.pdf (accessed on 25 September 2021).
- Vondrášek, J.; Mason, P.E.; Heyda, J.; Collins, K.D.; Jungwirth, P. The molecular origin of like-charge arginine—Arginine pairing in water. J. Phys. Chem. B 2009, 113, 9041–9045. [Google Scholar] [CrossRef]
- Vazdar, M.; Heyda, J.; Mason, P.E.; Tesei, G.; Allolio, C.; Lund, M.; Jungwirth, P. Arginine “magic”: Guanidinium Like-Charge Ion Pairing from Aqueous Salts to Cell Penetrating Peptides. Acc. Chem. Res. 2018, 51, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Kanduč, M.; Schneck, E.; Stubenrauch, C. Intersurfactant H-bonds between head groups of n-dodecyl-β-d-maltoside at the air-water interface. J. Colloid Interface Sci. 2021, 586, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, J.; Van Den Tempel, M. Dynamic measurements of dilational properties of a liquid interface. Chem. Eng. Sci. 1972, 27, 1283–1291. [Google Scholar] [CrossRef]
- Zamora, J.M.; Marquez, R.; Forgiarini, A.M.; Langevin, D.; Salager, J.L. Interfacial rheology of low interfacial tension systems using a new oscillating spinning drop method. J. Colloid Interface Sci. 2018, 519, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ravera, F.; Ferrari, M.; Santini, E.; Liggieri, L. Influence of surface processes on the dilational visco-elasticity of surfactant solutions. Adv. Colloid Interface Sci. 2005, 117, 75–100. [Google Scholar] [CrossRef]
- Loglio, G.; Kovalchuk, V.I.; Bykov, A.G.; Ferrari, M.; Krägel, J.; Liggieri, L.; Miller, R.; Noskov, B.A.; Pandolfini, P.; Ravera, F.; et al. Dynamic properties of mixed cationic/nonionic adsorbed layers at the n-hexane/water interface: Capillary pressure experiments under low gravity conditions. Colloids Interfaces 2018, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Lai, L.; Mei, P.; Wu, X.M.; Cheng, L.; Ren, Z.H.; Liu, Y. Interfacial Dynamic Properties and Dilational Rheology of Sulfonate Gemini Surfactant and its Mixtures with Quaternary Ammonium Bromides at the Air–Water Interface. J. Surfactants Deterg. 2017, 20, 565–576. [Google Scholar] [CrossRef]
- Noskov, B.A.; Alexandrov, D.A.; Miller, R. Dynamic surface elasticity of micellar and nonmicellar solutions of dodecyldimethyl phosphine oxide. Longitudinal wave study. J. Colloid Interface Sci. 1999, 219, 250–259. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. GAUSSIAN 09; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Case, D.A.; Babin, V.; Berryman, J.T.; Betz, R.M.; Cai, Q.; Cerutti, D.S.; Cheatham, T.E.; Darden, T.A.; Duke, R.E.; Gohlke, H.; et al. AMBER 14; University of California: San Francisco, CA, USA, 2014. [Google Scholar]
- Jarek, E.; Jasiński, T.; Barzyk, W.; Warszyński, P. The pH regulated surface activity of alkanoic acids. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 188–196. [Google Scholar] [CrossRef]









| Scheme | LAE Content | CMC (mM) | σCMC (mN/m) | Method | Reference |
|---|---|---|---|---|---|
| Mirenat-CF | 10.5% in propylene glycol | 4.9 | Isothermal titration calorimetry | [2] | |
| - | |||||
| Mirenat-CF | 10.5% in propylene glycol | 4.5 | Isothermal titration calorimetry | [9] | |
| - | |||||
| LAE Synthesized | - | 6.0 | 31.8 | Ring tensiometer | [16] |
| LAE (LAMIRSA) | 85–95% | 2.4 | 25.4 | Ring tensiometer | [17] |
| LAE.HCl (local supplier) | - | 0.9 | 25.5 | Conductivity, ring tensiometer | [18] |
| LAE synthesized | >95% | 6.2 | 30.2 | Ring tensiometer | [19,20] |
| Hydrolysis Path | Base Catalysed | Acid Catalysed | |||
|---|---|---|---|---|---|
| Data | ΔEt [kcal/mol] | ΔEh [kcal/mol] | ΔEt [kcal/mol] | ΔEh [kcal/mol] | |
| LAE → l-arginine ethyl ester + dodecanoate/dodecanoic acid | energy | 7.0 | −10.3 | 20.7 | 11.2 |
| enthalpy | 6.4 | −10.3 | 20.1 | 11.2 | |
| free energy | 17.5 | −15.6 | 31.3 | 9.0 | |
| LAE → LAS + ethanol | energy | −4.3 | −21.6 | 12.8 | 5.7 |
| enthalpy | −4.3 | −21.6 | 12.2 | 5.7 | |
| free energy | 4.9 | −24.2 | 23.8 | 4.4 | |
| Dimerization | Energy [kcal/mol] | Enthalpy [kcal/mol] | Free Energy [kcal/mol] |
|---|---|---|---|
| LAE-dodecanoate | −20.2 | −21.0 | −1.6 |
| LAE-LAS | −29.4 | −29.9 | −6.5 |
| Concentration [mM] | ||
|---|---|---|
| 0.2 | 30.1 | 0.0032 |
| 0.3 | 73.9 | 0.02 |
| 0.5 | 45.3 | 0.0081 |
| 0.8 | 26.9 | 0.03 |
| 1.0 | 12.6 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czakaj, A.; Jarek, E.; Krzan, M.; Warszyński, P. Ethyl Lauroyl Arginate, an Inherently Multicomponent Surfactant System. Molecules 2021, 26, 5894. https://doi.org/10.3390/molecules26195894
Czakaj A, Jarek E, Krzan M, Warszyński P. Ethyl Lauroyl Arginate, an Inherently Multicomponent Surfactant System. Molecules. 2021; 26(19):5894. https://doi.org/10.3390/molecules26195894
Chicago/Turabian StyleCzakaj, Agnieszka, Ewelina Jarek, Marcel Krzan, and Piotr Warszyński. 2021. "Ethyl Lauroyl Arginate, an Inherently Multicomponent Surfactant System" Molecules 26, no. 19: 5894. https://doi.org/10.3390/molecules26195894