Catalytic Oxidation of CO and Benzene over Metal Nanoparticles Loaded on Hierarchical MFI Zeolite
Abstract
:1. Introduction
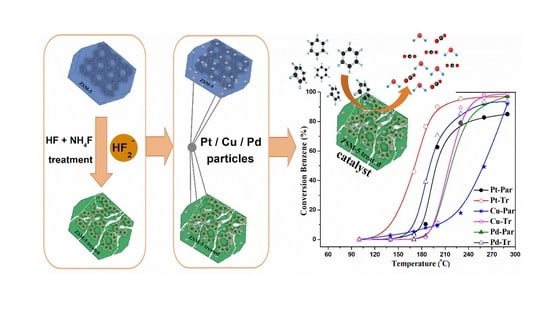
2. Results and Discussion
- (1)
- CuO + H2 → Cu0 + H2O
- (2)
- Cu2+ + 0.5H2 → Cu+ + H+
- (3)
- Cu+ + 0.5H2 → Cu0 + H+
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on human health. In Air Pollution and Control; Sharma, N., Agarwal, A., Eastwood, P., Gupta, T., Singh, A., Eds.; Energy, Environment and Sustainability; Springer: Singapore, 2018; pp. 119–142. [Google Scholar] [CrossRef]
- Ciccioli, P. VOCs and air pollution. In Chemistry and Analysis of Volatile Organic Compounds in the Environment; Bloemen, H.J.T., Burn, J., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 92–174. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Sekar, A.; Varghese, G.K.; Varma, M.K.R. Analysis of benzene air quality standards, monitoring methods and concentrations in indoor and outdoor environment. Heliyon 2019, 5, e02918. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, T.W.; Koh, H.L.; Lee, S.H.; Min, B.R. Complete benzene oxidation over Pt-Pd bimetal catalyst supported on γ-alumina: Influence of Pt-Pd ratio on the catalytic activity. Appl. Catal. A Gen. 2005, 280, 125–131. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Materials progress in the control of CO and CO2 emission at ambient conditions: An overview. Mater. Sci. Energy Technol. 2019, 2, 607–623. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Synthesis of CuMnOx catalysts by novel routes for selective catalytic oxidation of carbon monoxide. Comput. Toxicol. 2020, 16, 100132. [Google Scholar] [CrossRef]
- Khan, F.I.; Ghoshal, A.K. Removal of volatile organic compounds from polluted air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Kummer, J.T. Oxidation of CO and C2H4 by base metal catalysts prepared on honeycomb supports. In Catalysts for the Control of Automotive Pollutants; McEvoy, J.E., Ed.; American Chemical Society: Washington, DC, USA, 1975; Volume 143, pp. 178–192. [Google Scholar]
- Gaálová, J.; Jiratova, K.; Klempa, J.; Šolcová, O.; Maupin, I.; Mijoin, J.; Magnoux, P.; Barbier, J. Zeolite and mixed oxide catalysts for VOCs oxidation. WSEAS Trans. Environ. Dev. 2014, 10, 135–144. [Google Scholar]
- Dominguez, M.I.; Barrio, I.; Sanchez, M.; Centeno, M.A.; Montes, M.; Odriozola, J.A. CO and VOCs oxidation over Pt/SiO2 catalysts prepared using silicas obtained from stainless steel slags. Catal. Today 2008, 133–135, 467–474. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Ma, R.-H.; Lin, J.-J.; Meng, L.; Wang, Y.-J.; Luo, M.-F. Bifunctional Pd/Cr2O3-ZrO2 catalyst for the oxidation of volatile organic compounds. Ind. Eng. Chem. Res. 2011, 50, 10878–10882. [Google Scholar] [CrossRef]
- Tidahy, H.L.; Siffert, S.; Lamonier, J.-F.; Zhilinskaya, E.A.; Aboukais, A.; Yuan, Z.-Y.; Vantomme, A.; Su, B.-L.; Canet, X.; De Weireld, G.; et al. New Pd/hierarchical macro-mesoporous ZrO2, TiO2 and ZrO2-TiO2 catalysts for VOCs total oxidation. Appl. Catal. A Gen. 2006, 310, 61–69. [Google Scholar] [CrossRef]
- Huang, H.; Huang, W.; Xu, Y.; Ye, X.; Wu, M.; Shao, Q.; Ou, G.; Peng, Z.; Shi, J.; Chen, J.; et al. Catalytic oxidation of gaseous benzene with ozone over zeolite-supported metal oxide nanoparticles at room temperature. Catal. Today 2015, 258, 627–633. [Google Scholar] [CrossRef]
- Barakat, T.; Rooke, J.C.; Tidahy, H.L.; Hosseini, M.; Cousin, R.; Lamonier, J.-F.; Giraudon, J.-M.; De Weireld, G.; Su, B.-L.; Siffert, S. Noble-metal-based catalysts supported on zeolites and macro-mesoporous metal oxide supports for the total oxidation of volatile organic compounds. ChemSusChem. 2011, 4, 1420–1430. [Google Scholar] [CrossRef]
- International Zeolite Association. Database of Zeolite Structures—MFI. Available online: http://europe.iza-structure.org/IZA-SC/framework.php?STC=MFI (accessed on 1 July 2021).
- Díaz, I.; Kokkoli, E.; Terasaki, O.; Tsapatsis, M. Surface Structure of Zeolite (MFI) Crystals. Chem. Mater. 2004, 16, 5226–5232. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Song, Y.; Sun, C.; Shen, W.; Lin, L. Hydrothermal post-synthesis of HZSM-5 zeolite to enhance the coke-resistance of Mo/HZSM-5 catalyst for methane dehydroaromatization reaction: Reconstruction of pore structure and modification of acidity. Appl. Catal. A Gen. 2007, 317, 266–274. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.Z.; Tang, C.; Zhang, Y.H. Modifications of ZSM-5 zeolites and their applications in catalytic degradation of LDPE. Polym. Degrad. Stab. 2003, 80, 23–30. [Google Scholar] [CrossRef]
- Sato, H.; Ishii, N.; Hirose, K.; Nakamura, S. Some catalytic applications of ZSM-5 zeolite: Para-selective dealkylation and vapor phase Beckmann rearrangement. In Studies in Surface Science and Catalysis; Murakami, Y., Iijima, A., Ward, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 755–762. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Wang, G.; Sun, Y.; Cheng, J.; Zhang, Z.; Zhang, X.; Hao, Z. Fluorine-enhanced Pt-ZSM-5 catalysts for low-temperature oxidation of ethylene. Catal. Sci. Technol. 2018, 8, 1988–1996. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Cibrián, S.; Gutiérrez-Ortiz, J.I.; González-Velasco, J.R. Effect of Pd addition on the catalytic performance of H-ZSM-5 zeolite in chlorinated VOCs combustion. In Studies in Surface Science and Catalysis; Aiello, R., Giordano, G., Testa, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 142, pp. 847–854. [Google Scholar] [CrossRef]
- Chen, H.; Rui, Z.; Wang, X.; Ji, H. Multifunctional Pt/ZSM-5 catalyst for complete oxidation of gaseous formaldehyde at ambient temperature. Catal. Today 2015, 258, 56–63. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Li, M.; Ran, R. Roles of acid sites on Pt/H-ZSM5 catalyst in catalytic oxidation of diesel soot. ACS Catal. 2015, 5, 909–919. [Google Scholar] [CrossRef]
- He, C.; Li, P.; Cheng, J.; Wang, H.; Li, J.; Li, Q.; Hao, Z. Synthesis and characterization of Pd/ZSM-5/MCM-48 biporous catalysts with superior activity for benzene oxidation. Appl. Catal. A Gen. 2010, 382, 167–175. [Google Scholar] [CrossRef]
- Xing, X.; Li, N.; Sun, Y.; Wang, G.; Cheng, J.; Hao, Z. Selective catalytic oxidation of n-butylamine over Cu-zeolite catalysts. Catal. Today 2020, 339, 192–199. [Google Scholar] [CrossRef]
- Xing, X.; Li, N.; Cheng, J.; Sun, Y.; Zhang, Z.; Zhang, X.; Hao, Z. Synergistic effects of Cu species and acidity of Cu-ZSM-5 on catalytic performance for selective catalytic oxidation of n-butylamine. J. Environ. Sci. 2020, 96, 55–63. [Google Scholar] [CrossRef]
- Nanba, T.; Masukawa, S.; Ogata, A.; Uchisawa, J.; Obuchi, A. Active sites of Cu-ZSM-5 for the decomposition of acrylonitrile. Appl. Catal. B Environ. 2005, 61, 288–296. [Google Scholar] [CrossRef]
- Vovchok, D.; Tata, J.; Orozco, I.; Zhang, F.; Palomino, R.M.; Xu, W.; Harper, L.; Khatib, S.J.; Rodriguez, J.A.; Senanayake, S.D. Location and chemical speciation of Cu in ZSM-5 during the water-gas shift reaction. Catal. Today 2019, 323, 216–224. [Google Scholar] [CrossRef]
- Viswanadham, N.; Saxena, S.K.; Al-Muhtase, A.H. Cu functionalized nano crystalline ZSM-5 as efficient catalyst for selective oxidation of toluene. Mater. Today Chem. 2017, 3, 37–48. [Google Scholar] [CrossRef]
- Schay, Z.; Guczi, L. Decomposition of NO over Cu-ZSM-5 Zeolites. A Transient Kinetic Study. Catal. Today 1993, 17, 175–180. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, S.; Wang, C.; Li, J.; Xiao, F.S.; Qi, C. Pd/transition metal oxides functionalized ZSM-5 single crystals with b-axis aligned mesopores: Efficient and long-lived catalysts for benzene combustion. Appl. Catal. B Environ. 2014, 148–149, 106–113. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, M.; Mo, S.; Cheng, H.; Fu, M.; Chen, P.; Chen, L.; Shi, W.; Ye, D. Catalytic performance of toluene combustion over Pt nanoparticles supported on pore-modified macro-meso-microporous zeolite foam. Nanomaterials 2020, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Martínez, A.; Arroyo, P.A.; Monteiro, J.L.F.; Sousa-Aguiar, E.F. Isobutane/2-butene alkylation on zeolite beta: Influence of post-synthesis treatments. Appl. Catal. A Gen. 1996, 142, 139–150. [Google Scholar] [CrossRef]
- Müller, M.; Harvey, G.; Prins, R. Comparison of the dealumination of zeolites beta, mordenite, ZSM-5 and ferrierite by thermal treatment, leaching with oxalic acid and treatment with SiCl4 by 1H, 29Si and 27Al MAS NMR. Microporous Mesoporous Mater. 2000, 34, 135–147. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Holm, M.S.; Olsbye, U.; Lillerud, K.P.; Svelle, S. Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A Gen. 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, F.; Xiao, G. Performance of hierarchical HZSM-5 zeolites prepared by NaOH treatments in the aromatization of glycerol. RSC Adv. 2015, 5, 63697–63704. [Google Scholar] [CrossRef]
- Van Laak, A.N.C. Post-Synthesis Modifications on Zeolites for Improved Accessibility and Catalytic Performance. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2011. [Google Scholar]
- Yu, L.; Huang, S.; Miao, S.; Chen, F.; Zhang, S.; Liu, Z.; Xie, S.; Xu, L. A facile top-down protocol for postsynthesis modification of hierarchical aluminum-rich MFI zeolites. Chem. Eur. J. 2015, 21, 1048–1054. [Google Scholar] [CrossRef]
- Qin, Z.; Lakiss, L.; Gilson, J.P.; Thomas, K.; Goupil, J.M.; Fernandez, C.; Valtchev, V. Chemical equilibrium-controlled etching of MFI-type zeolite and its influence on zeolite structure, acidity, and catalytic activity. Chem. Mater. 2013, 25, 2759–2766. [Google Scholar] [CrossRef]
- Knotter, D.M. The Chemistry of Wet Cleaning. In Handbook of Cleaning in Semiconductor Manufacturing; Reinhardt, K.A., Reidy, R.F., Eds.; Wiley-Scrivener: Hoboken, NJ, USA, 2010; Volume 3, pp. 39–94. [Google Scholar] [CrossRef]
- Qin, Z.; Gilson, J.P.; Valtchev, V. Mesoporous zeolites by fluoride etching. Curr. Opin. Chem. Eng. 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Todorova, T.; Vimont, A.; Ruaux, V.; Qin, Z.; Gilson, J.P.; Valtchev, V. In situ and post-synthesis control of physiochemical properties of FER-type Crystals. Microporous Mesoporous Mater. 2014, 200, 334–342. [Google Scholar] [CrossRef]
- Kalvachev, Y.; Todorova, T.; Nihtianova, D.; Lazarova, H.; Popova, M. Fluoride etching of mordenite and its influence on catalytic activity. J. Mater. Sci. 2017, 9, 5297–5308. [Google Scholar] [CrossRef]
- Todorova, T.; Kalvachev, Y.; Lazarova, H.; Popova, M. Catalytic activity of modified mordenite in the reaction of m-xylene transformation. C. R. Acad. Bul. Sci. 2016, 69, 1283–1290. [Google Scholar]
- Kalvachev, Y.; Todorova, T.; Popov, C. Recent Progress in synthesis and application of nanosized and hierarchical mordenite—A Short Review. Catalysts 2021, 11, 308. [Google Scholar] [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V.D.B.C.; Likozar, B. Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification. Catal. Commun. 2017, 100, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Todorova, T.; Shestakova, P.; Petrova, T.; Popova, M.; Lazarova, H.; Kalvachev, Y. Fluoride etching of AlZSM-5 and GaZSM-5 zeolites. J. Mater. Sci. 2020, 55, 13799–13814. [Google Scholar] [CrossRef]
- Garces, H.; Roller, J.; King’ondu, C.; Dharmarathna, S.; Ristau, R.; Jain, R.; Maric, R.; Suib, S. Formation of platinum (Pt) nanocluster coatings on K-OMS-2 manganese oxide membranes by reactive spray deposition technique (RSDT) for extended stability during CO oxidation. Adv. Chem. Eng. Sci. 2014, 4, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Improved electrode performance in microbial fuel cells and the enhanced visible light-induced photoelectrochemical behavior of PtOx@M-TiO2 nanocomposites. Ceram. Int. 2015, 41, 9131–9139. [Google Scholar] [CrossRef]
- Bouazizi, N.; Bargougui, R.; Oueslati, A.; Benslama, R. Effect of synthesis time on structural, optical and electrical properties of CuO nanoparticles synthesized by reflux condensation method. Adv. Mater. Lett. 2015, 6, 158–164. [Google Scholar] [CrossRef]
- Lee, D.W.; Jin, M.H.; Lee, Y.J.; Park, J.-H.; Lee, C.-B.; Park, J.-S. Reducing-agent-free instant synthesis of carbon-supported Pd catalysts in a green leidenfrost droplet reactor and catalytic activity in formic acid dehydrogenation. Sci. Rep. 2016, 6, 26474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeshbabu, E.; Rao, G.R. High electrocatalytic activity of Pt/C catalyst promoted by TT-Nb2O5 nanoparticles under acidic conditions. Chem. Sel. 2017, 2, 4204–4212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Qiu, A.; Wang, Y.; Xu, Y.; Wu, P. Propane dehydrogenation on PtSn/ZSM-5 catalyst: Effect of tin as a promoter. Catal. Commun. 2006, 7, 860–866. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Huang, L.; Zhou, S.; Sheng, X.; Wang, Q.; Zhang, C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation. Chem. Eng. J. 2015, 270, 352–361. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Li, Y.; Wang, Q.; Xu, H.; Chen, Y. Promotion of catalytic performance by adding Cu into Pt/ZSM-5 catalyst for selective catalytic oxidation of ammonia. J. Taiwan Inst. Chem. Eng. 2017, 78, 401–408. [Google Scholar] [CrossRef]
- Sangeetha, P.; Shanthi, K.; Rama Rao, K.S.; Viswanathan, B.; Selvam, P. Hydrogenation of nitrobenzene over palladium-supported catalysts—Effect of support. Appl. Catal. A Gen. 2009, 353, 160–165. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, Q.-H.; Shen, S.-C.; Kawi, S.; Hidajat, K. Catalytic oxidation of pyridine on the supported copper catalysts in the presence of excess oxygen. J. Catal. 2004, 225, 128–137. [Google Scholar] [CrossRef]
- Urquieta-González, E.A.; Martins, L.; Peguin, R.P.S.; Batista, M.S. Identification of extra-framework species on Fe/ZSM-5 and Cu/ZSM-5 catalysts typical microporous molecular sieves with zeolitic structure. Mater. Res. 2002, 5, 321–327. [Google Scholar] [CrossRef]
- Huang, T.J.; Tsai, D.H. CO oxidation behavior of copper and copper oxides. Catal. Lett. 2003, 87, 173–178. [Google Scholar] [CrossRef]





| Sample | SBET a m2/g | Smi b m2/g | Vt c cm3/g | Vmi b cm3/g | Vsec d cm3/g | Dav e nm |
|---|---|---|---|---|---|---|
| Par | 290 | 242 | 0.22 | 0.13 | 0.09 | 3.0 |
| Tr | 270 | 212 | 0.36 | 0.11 | 0.25 | 5.4 |
| Pt-Par | 328 | 277 | 0.23 | 0.15 | 0.08 | 2.8 |
| Pt-Tr | 361 | 294 | 0.34 | 0.15 | 0.19 | 3.8 |
| Cu-Par | 297 | 255 | 0.20 | 0.14 | 0.06 | 2.8 |
| Cu-Tr | 319 | 272 | 0.24 | 0.14 | 0.10 | 3.1 |
| Pd-Par | 318 | 269 | 0.22 | 0.14 | 0.08 | 2.8 |
| Pd-Tr | 344 | 276 | 0.32 | 0.14 | 0.18 | 3.7 |
| Sample | 90% CO Conv. (T °C) | 90% C6H6 Conv. (T °C) |
|---|---|---|
| Pt-Par | 211 | - |
| Pt-Tr | 189 | 206 |
| Cu-Par | 191 | 289 |
| Cu-Tr | 234 | 242 |
| Pd-Par | 217 | 258 |
| Pd-Tr | 184 | 249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, T.; Petrova, P.; Kalvachev, Y. Catalytic Oxidation of CO and Benzene over Metal Nanoparticles Loaded on Hierarchical MFI Zeolite. Molecules 2021, 26, 5893. https://doi.org/10.3390/molecules26195893
Todorova T, Petrova P, Kalvachev Y. Catalytic Oxidation of CO and Benzene over Metal Nanoparticles Loaded on Hierarchical MFI Zeolite. Molecules. 2021; 26(19):5893. https://doi.org/10.3390/molecules26195893
Chicago/Turabian StyleTodorova, Totka, Petya Petrova, and Yuri Kalvachev. 2021. "Catalytic Oxidation of CO and Benzene over Metal Nanoparticles Loaded on Hierarchical MFI Zeolite" Molecules 26, no. 19: 5893. https://doi.org/10.3390/molecules26195893