Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Activation of GO/MR Composite
2.3. Characterization
2.4. Adsorption Experiment
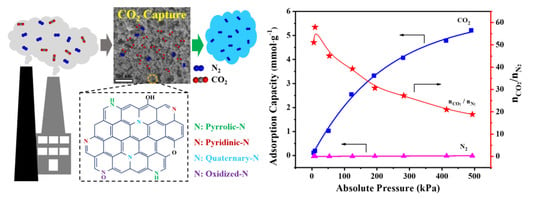
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kang, S.; Hwang, J. Fabrication of hollow activated carbon nanofibers (HACNFs) containing manganese oxide catalyst for toluene removal via two-step process of electrospinning and thermal treatment. Chem. Eng. J. 2020, 379, 122315. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, T.C. N,S-containing polycondensate-derived porous carbon materials for superior CO2 adsorption and supercapacitor. Appl. Surf. Sci. 2021, 562, 150128. [Google Scholar] [CrossRef]
- Huck, J.M.; Lin, L.-C.; Berger, A.H.; Shahrak, M.N.; Martin, R.L.; Bhown, A.S.; Haranczyk, M.; Reuter, K.; Smit, B. Evaluating different classes of porous materials for carbon capture. Energy Environ. Sci. 2014, 7, 4132–4146. [Google Scholar] [CrossRef]
- Nie, L.; Mu, Y.; Jin, J.; Chen, J.; Mi, J. Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas. Chin. J. Chem. Eng. 2018, 26, 2303–2317. [Google Scholar] [CrossRef]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Jiang, H.; Yuan, S. MWCNT Decorated rich N-doped porous carbon with tunable porosity for CO2 capture. Molecules 2021, 26, 3451. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-a review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Sun, Y. Carbon-based adsorbents for post-combustion capture: A review. Greenh. Gases Sci. Technol. 2018, 8, 11–36. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Hu, G.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous carbons: Structure-oriented design and versatile applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.-G.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef]
- Yue, L.; Xia, Q.; Wang, L.; Wang, L.; DaCosta, H.; Yang, J.; Hu, X. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell. J. Colloid Interface Sci. 2018, 511, 259–267. [Google Scholar] [CrossRef]
- Wang, L.; Rao, L.; Xia, B.; Wang, L.; Yue, L.; Liang, Y.; DaCosta, H.; Hu, X. Highly efficient CO2 adsorption by nitrogen-doped porous carbons synthesized with low-temperature sodium amide activation. Carbon 2018, 130, 31–40. [Google Scholar] [CrossRef]
- Shao, L.; Liu, M.; Huang, J.; Liu, Y.-N. CO2 capture by nitrogen-doped porous carbons derived from nitrogen-containing hyper-cross-linked polymers. J. Colloid Interface Sci. 2018, 513, 304–313. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. N-Doped porous carbon derived from solvent-free synthesis of cross-linked triazine polymers for simultaneously achieving CO2 capture and supercapacitors. Chem. Eur. J. 2021, 27, 7908–7914. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Ma, R.; Liu, S.; Wang, L.; Wu, Z.; Yang, J.; Hu, X. Nitrogen enriched porous carbons from d-glucose with excellent CO2 capture performance. Chem. Eng. J. 2019, 362, 794–801. [Google Scholar] [CrossRef]
- Rao, L.; Liu, S.; Wang, L.; Ma, C.; Wu, J.; An, L.; Hu, X. N-doped porous carbons from low-temperature and single-step sodium amide activation of carbonized water chestnut shell with excellent CO2 capture performance. Chem. Eng. J. 2019, 359, 428–435. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Zhang, F.; Li, Q.; Zhu, G. One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol–urea–formaldehyde resin for CO2 capture. Carbon 2014, 69, 502–514. [Google Scholar] [CrossRef]
- Mu, J.-J.; He, C.-Y.; Sun, W.-J.; Guan, Y. Selective synthesis of three-dimensional ZnO@Ag/SiO2@Ag nanorod arrays as surface-enhanced Raman scattering substrates with tunable interior dielectric layer. Chin. Phys. B 2019, 28, 124204. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Muhammad, Y.; Subhan, S.; Zhang, Y.; Ling, Y.; Li, J.; Zhao, Z.; Zhao, Z. Pyrrolic N-enriched carbon fabricated from dopamine-melamine via fast mechanochemical copolymerization for highly selective separation of CO2 from CO2/N2. Chem. Eng. J. 2018, 349, 92–100. [Google Scholar] [CrossRef]
- Chen, C.; Yu, Y.; He, C.; Wang, L.; Huang, H.; Albilali, R.; Cheng, J.; Hao, Z. Efficient capture of CO2 over ordered micro-mesoporous hybrid carbon nanosphere. Appl. Surf. Sci. 2018, 439, 113–121. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdelnaby, A.M.; Alloush, N.A.A.; Qasem, B.A.; Al-Maythalony, R.B.; Mansour, K.E.; Cordova, O.C.S.; Al-Hamouz, O.C.S. Carbon dioxide capture in the presence of water by an amine-based crosslinked porous polymer. J. Mater. Chem. A 2018, 6, 6455–6462. [Google Scholar]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A controllable synthesis of rich nitrogen-doped ordered mesoporous xarbon for CO2 capture and supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, Y.; Ding, J.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.; Deng, S. A new choice of polymer precursor for solvent-free method: Preparation of N-enriched porous carbons for highly selective CO2 capture. Chem. Eng. J. 2019, 355, 963–973. [Google Scholar] [CrossRef]
- Ashourirad, B.; Sekizkardes, A.K.; Altarawneh, S.; El-Kaderi, H.M. Exceptional gas adsorption properties by nitrogen-doped porous carbons derived from benzimidazole-linked polymers. Chem. Mater. 2015, 27, 1349–1358. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, P.-W.; Chen, Q.; Fu, C.; Han, B.-H. Polycarbazole and biomass-derived flexible nitrogen-doped porous carbon materials for gas adsorption and sensing. J. Mater. Chem. A 2020, 8, 6804–6811. [Google Scholar] [CrossRef]
- Shao, L.; Sang, Y.; Huang, J.; Liu, Y.-N. Triazine-based hyper-cross-linked polymers with inorganic-organic hybrid framework derived porous carbons for CO2 capture. Chem. Eng. J. 2018, 353, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.-F.; Zhong, J.-J.; Qian, X.; Song, S.-L.; Zhang, Y.-G.; Li, D.-H. Nitrogen-enriched porous polyacrylonitrile-based carbon fibers for CO2 capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. [Google Scholar] [CrossRef]
- Chen, C.; Huang, H.; Yu, Y.; Shi, J.; He, C.; Albilali, R.; Pan, H. Template-free synthesis of hierarchical porous carbon with controlled morphology for CO2 efficient capture. Chem. Eng. J. 2018, 353, 584–594. [Google Scholar] [CrossRef]
- Saleh, M.; Tiwari, J.N.; Kemp, K.C.; Yousuf, M.; Kim, K.S. Highly selective and stable carbon dioxide uptake in polyindole-derived microporous carbon materials. Environ. Sci. Technol. 2013, 47, 5467–5473. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Park, S.-J. From chitosan to urea-modified carbons: Tailoring the ultra-microporosity for enhanced CO2 adsorption. Carbon 2020, 159, 625–637. [Google Scholar] [CrossRef]
- Yue, L.; Rao, L.; Wang, L.; An, L.; Hou, C.; Ma, C.; DaCosta, H.; Hu, X. Efficient CO2 adsorption on nitrogen-doped porous carbons derived from d-glucose. Energy Fuels 2018, 32, 6955–6963. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C.; Juang, R.-S. Adsorption of CO2 at atmospheric pressure on activated carbons prepared from melamine-modified phenol–formaldehyde resins. Sep. Purif. Technol. 2015, 140, 53–60. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. rGO/N-porous carbon composites for enhanced CO2 capture and energy storage performances. J. Alloys Compd. 2021, 857, 157534. [Google Scholar] [CrossRef]
- Sevilla, M.; Díez, N.; Fuertes, A.B. More sustainable chemical activation strategies for the production of porous carbons. ChemSusChem 2021, 14, 94–117. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, T.C.; Yuan, S.; Liang, B. N-doped porous carbon derived from rGO-Incorporated polyphenylenediamine composites for CO2 adsorption and supercapacitors. J. Power Sources 2020, 472, 228610. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Rayalu, S.S. Melamine-based resins and their carbons for CO2 capture: A review. Emerg. Mater. 2021, 4, 545–563. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.J.; Lim, S.Y.; Kim, B.G.; Choi, J.W. Combined CO2-philicity and ordered mesoporosity for highly selective CO2 capture at high temperatures. J. Am. Chem. Soc. 2015, 137, 7210–7216. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.-J. Tunable nitrogen-doped microporous carbons: Delineating the role of optimum pore size for enhanced CO2 adsorption. Chem. Eng. J. 2019, 362, 731–742. [Google Scholar] [CrossRef]
- Song, X.; Chen, Q.; Shen, E.; Liu, H. N-doped 3D hierarchical carbon from resorcinol–formaldehyde–melamine resin for high-performance supercapacitors. New J. Chem. 2020, 44, 8638–8649. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Pan, N.; Li, L.; Ding, J.; Li, S.; Wang, R.; Jin, Y.; Wang, X.; Xia, C. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J. Hazard. Mater. 2016, 309, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Shi, W.; Han, S.; Zhou, Y.; Liu, Y.; Zhang, S.; Yang, B. N-rich porous carbons with a high graphitization degree and multiscale pore network for boosting high-rate supercapacitor with ultrafast charging. Chem. Eng. J. 2018, 350, 585–598. [Google Scholar] [CrossRef]
- Lin, Z.; Yao, Y.; Li, Z.; Liu, Y.; Li, Z.; Wong, C.-P. Solvent-assisted thermal reduction of graphite oxide. J. Phys. Chem. C 2010, 114, 14819–14825. [Google Scholar] [CrossRef]
- Geng, J.; Yin, Y.; Liang, Q.; Zhu, Z.; Luo, H. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism. Chem. Eng. J. 2019, 361, 1497–1510. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.; Muhammad, F.; Wang, A.; Yu, G.; Ma, H.; Zhu, G. Simple fabrication of an ordered nitrogen-doped mesoporous carbon with resorcinol–melamine–formaldehyde resin. Microporous Mesoporous Mater. 2014, 190, 117–127. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, S.; Su, H.; Wang, M.; Qiao, W.; Ling, L.; Long, D. Facile preparation and ultra-microporous structure of melamine–resorcinol–formaldehyde polymeric microspheres. Chem. Commun. 2013, 49, 3763–3765. [Google Scholar] [CrossRef]
- Goel, C.; Bhunia, H.; Bajpai, P.K. Synthesis of nitrogen doped mesoporous carbons for carbon dioxide capture. RSC Adv. 2015, 5, 46568–46582. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Ding, S.; Dai, S. One-step synthesis of nitrogen-doped graphene-like meso-macroporous carbons as highly efficient and selective adsorbents for CO2 capture. J. Mater. Chem. A 2016, 4, 14567–14571. [Google Scholar] [CrossRef]
- Meng, L.; Yin, W.; Wang, S.; Wu, X.; Hou, J.; Yin, W.; Feng, K.; Ok, Y.S.; Wang, X. Photocatalytic behavior of biochar-modified carbon nitride with enriched visible-light reactivity. Chemosphere 2020, 239, 124713. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- László, K.; Tombácz, E.; Josepovits, K. Effect of activation on the surface chemistry of carbons from polymer precursors. Carbon 2001, 39, 1217–1228. [Google Scholar] [CrossRef]
- Kundu, S.; Xia, W.; Busser, W.; Becker, M.; Schmidt, D.A.; Havenith, M.; Muhler, M. The formation of nitrogen-containing functional groups on carbon nanotube surfaces: A quantitative XPS and TPD study. Phys. Chem. Chem. Phys. 2010, 12, 4351–4359. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel difunctional material for enhancing CO2 capture and supercapacitance. J. Mater. Sci. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Pietrzak, R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Schlögl, R.; Su, D.S. Dynamic surface rearrangement and thermal stability of nitrogen functional groups on carbon nanotubes. Chem. Commun. 2008, 4891–4893. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Influence of porous texture and surface chemistry on the CO2 adsorption capacity of porous carbons: Acidic and basic site interactions. ACS Appl. Mater. Interfaces 2014, 6, 21237–21247. [Google Scholar] [CrossRef] [PubMed]








| Sample | Textural Properties | Chemical Composition | CO2 Adsorbed Amount (mmol·g−1) | |||||
|---|---|---|---|---|---|---|---|---|
| SBET a (m2·g−1) | Vp b (cm3·g−1) | Average Pore Size (nm) | N (wt.%) | C (wt.%) | O (wt.%) | 100 kPa | 500 kPa | |
| GO | 450 | 2.20 | 20.8 | - | - | - | 0.18 | 0.41 |
| MR | 0.99 | 0.003 | - | - | - | - | 0.13 | 0.32 |
| MR-500 | 204 | 0.10 | 3.55 | 7.11 | 76.15 | 16.74 | 0.19 | 0.49 |
| GO(0.25)/MR | 6.7 | 0.008 | 8.50 | 7.30 | 67.14 | 25.56 | 0.40 | 0.76 |
| GO(0.25)/MR-400 | 32 | 0.06 | 12.70 | 6.79 | 77.93 | 15.28 | 0.46 | 1.05 |
| GO(0.25)/MR-500 | 1264 | 0.47 | 3.90 | 6.92 | 80.09 | 12.99 | 2.27 | 5.21 |
| GO(0.25)/MR-600 | 972 | 0.67 | 5.70 | 6.55 | 82.42 | 13.23 | 1.33 | 4.29 |
| GO(0.25)/MR-700 | 790 | 0.28 | 3.80 | 3.42 | 82.85 | 13.73 | 0.87 | 1.89 |
| GO(0.375)/MR-500 | 784 | 0.39 | 3.16 | 6.84 | 81.79 | 11.37 | 0.74 | 2.81 |
| Model | Fitted Parameters | Temperature (K) | |||
|---|---|---|---|---|---|
| 298.15 | 308.15 | 318.15 | 323.15 | ||
| Freundlich | KF (mmol·g−1·kPa−n) | 0.1623 | 0.1343 | 0.0663 | 0.0548 |
| n | 1.7717 | 1.7581 | 1.5050 | 1.4678 | |
| χ2 (×10−4) | 329.9 | 205.6 | 256.8 | 166.5 | |
| R2 | 0.9912 | 0.9924 | 0.9890 | 0.9916 | |
| Langmuir | Qmax (mmol·g−1) | 5.1950 | 4.4054 | 3.9498 | 3.6210 |
| KL (kPa−1) | 0.0041 | 0.0040 | 0.0025 | 0.0022 | |
| χ2 (×10−4) | 44.2 | 68.6 | 46.2 | 24.4 | |
| R2 | 0.9988 | 0.9975 | 0.9980 | 0.9988 | |
| Redlich–Peterson | aR (kPa−bR) | 0.0212 | 0.0370 | 0.0033 | 0.0031 |
| KR (mmol·g−1·kPa−n) | 0.0411 | 0.0391 | 0.0182 | 0.0158 | |
| bR | 0.7928 | 0.7262 | 0.9600 | 0.9551 | |
| χ2 (×10−4) | 6.3 | 11.7 | 55 | 29 | |
| R2 | 0.9998 | 0.9996 | 0.9976 | 0.9985 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, L.; Xiao, J.; Jiang, H.; Yuan, S. Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption. Molecules 2021, 26, 5293. https://doi.org/10.3390/molecules26175293
Ouyang L, Xiao J, Jiang H, Yuan S. Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption. Molecules. 2021; 26(17):5293. https://doi.org/10.3390/molecules26175293
Chicago/Turabian StyleOuyang, Like, Jianfei Xiao, Housheng Jiang, and Shaojun Yuan. 2021. "Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption" Molecules 26, no. 17: 5293. https://doi.org/10.3390/molecules26175293