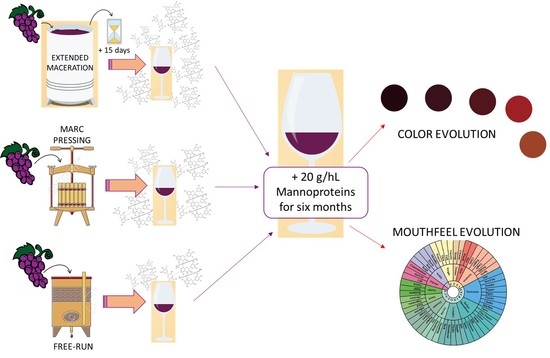
Commercial Mannoproteins Improve the Mouthfeel and Colour of Wines Obtained by Excessive Tannin Extraction
Abstract
:1. Introduction
2. Results
2.1. The Content in BSA-Reactive Tannins and Vanillin-Reactive Flavans
2.2. The Effect of Mannoproteins on the Colour of Wines
2.3. The Effect of Mannoproteins on the Mouthfeel of Wines
2.4. Relationships between Subqualities, Colour Parameters, and Phenolic Content of Wines
2.5. The Effect of Mannoproteins on Aroma and Odour Descriptors
3. Discussion
4. Materials and Methods
4.1. Wine Samples
4.2. Yeast Mannoprotein Products
4.3. Wine Sensory Evaluation
4.4. Chemical Analyses
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds during Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Tao, Y.S.; Dizy, M.; Ferreira, V.; Fernández-Zurbano, P. Relationship between Nonvolatile Composition and Sensory Properties of Premium Spanish Red Wines and Their Correlation to Quality Perception. J. Agric. Food Chem. 2010, 58, 12407–12416. [Google Scholar] [CrossRef] [PubMed]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors Affecting Extraction and Evolution of Phenolic Compounds during Red Wine Maceration and the Role of Process Modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Nel, A.P.; van Rensburg, P.; Lambrechts, M.G. The Influence of Different Winemaking Techniques on the Extraction of Grape Tannins and Anthocyanins. S. Afr. J. Enol. Vitic. 2016, 35, 304–320. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Frost, S.C.; Blackman, J.W.; Ebeler, S.E.; Heymann, H. Analysis of Temporal Dominance of Sensation Data Using Correspondence Analysis on Merlot Wine with Differing Maceration and Cap Management Regimes. Food Qual. Prefer. 2018, 64, 245–252. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Moio, L. Use of Patatin, a Protein Extracted from Potato, as Alternative to Animal Proteins in Fining of Red Wine. Eur. Food Res. Technol. 2012, 235, 753–765. [Google Scholar] [CrossRef]
- Rinaldi, A.; Errichiello, F.; Moio, L. Alternative Fining of Sangiovese Wine: Effect on Phenolic Substances and Sensory Characteristics. Aust. J. Grape Wine Res. 2021, 27, 128–137. [Google Scholar] [CrossRef]
- McRae, J.M.; Schulkin, A.; Kassara, S.; Holt, H.E.; Smith, P.A. Sensory Properties of Wine Tannin Fractions: Implications for in-Mouth Sensory Properties. J. Agric. Food Chem. 2013, 61, 719–727. [Google Scholar] [CrossRef]
- Rinaldi, A.; Louazil, P.; Iturmendi, N.; Moine, V.; Moio, L. Effect of Marc Pressing and Geographical Area on Sangiovese Wine Quality. Lebenson. Wiss. Technol. 2020, 118. [Google Scholar] [CrossRef]
- Lukić, I.; Milicević, B.; Banović, M.; Tomas, S.; Radeka, S.; Persurić, D. Characterization and Differentiation of Monovarietal Grape Marc Distillates on the Basis of Varietal Aroma Compound Composition. J. Agric. Food Chem. 2010, 58, 7351–7360. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva Di Troia Grapes by Aromatic Series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The Mouthfeel Properties of Polysaccharides and Anthocyanins in a Wine like Medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Charpentier, C.; Feuillat, M. Yeast Autolysis G. In Wine Microbiology and Biotechnology; Fleet, H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993. [Google Scholar]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of Non-Saccharomyces Wine Yeasts as Novel Sources of Mannoproteins in Wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Coppola, M.; Moio, L. Aging of Aglianico and Sangiovese Wine on Mannoproteins: Effect on Astringency and Colour. Lebenson. Wiss. Technol. 2019, 105, 233–241. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, D.; Cebollero, E.; Gonzalez, R. A Recombinant Saccharomyces Cerevisiae Strain Overproducing Mannoproteins Stabilizes Wine against Protein Haze. Appl. Environ. Microbiol. 2008, 74, 5533–5540. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.; Fernandes, C.; Nunes, F.M.; Filipe-Ribeiro, L.; Cosme, F. Influence of the Structural Features of Commercial Mannoproteins in White Wine Protein Stabilization and Chemical and Sensory Properties. Food Chem. 2014, 159, 47–54. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Martínez-Lapuente, L.; Bueno-Herrera, M.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Use of Commercial Dry Yeast Products Rich in Mannoproteins for White and Rosé Sparkling Wine Elaboration. J. Agric. Food Chem. 2015, 63, 5670–5681. [Google Scholar] [CrossRef]
- Manjón, E.; Brás, N.F.; García-Estévez, I.; Escribano-Bailón, M.T. Cell Wall Mannoproteins from Yeast Affect Salivary Protein-Flavanol Interactions through Different Molecular Mechanisms. J. Agric. Food Chem. 2020, 68, 13459–13468. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration Enzymes and Mannoproteins: A Possible Strategy to Increase Colloidal Stability and Colour Extraction in Red Wines. J. Agric. Food Chem. 2007, 55, 4854–4862. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces Strains on the Quality of Red Wines Aged on Lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Polysaccharide Characterization of Commercial Dry Yeast Preparations and Their Effect on White and Red Wine Composition. Lebenson. Wiss. Technol. 2012, 48, 215–223. [Google Scholar] [CrossRef]
- Marchal, A.; Marullo, P.; Moine, V.; Dubourdieu, D. Influence of Yeast Macromolecules on Sweetness in Dry Wines: Role of the Saccharomyces Cerevisiae Protein Hsp12. J. Agric. Food Chem. 2011, 59, 2004–2010. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Moio, L. Evolution of Sangiovese Wines with Varied Tannin and Anthocyanin Ratios during Oxidative Aging. Front. Chem. 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of Polysaccharides by Yeasts and the Influence of Released Polysaccharides on Colour Stability and Wine Astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Rinaldi, A.; Moio, L. Effect of Enological Tannin Addition on Astringency Subqualities and Phenolic Content of Red Wines. J. Sens. Stud. 2018, 33, e12325. [Google Scholar] [CrossRef]
- Saucier, C.; Glories, Y.; Roux, D. Interactions Tanins-Colloides: Nouvelles Avancées Concernant La Notion de « bons » et de « mauvais » tanins. Revue des Œnologues 2000, 94, 7–8. [Google Scholar]
- Maury, C.; Sarni-Manchado, P.; Lefebvre, S.; Cheynier, V.; Moutounet, M. Influence of Fining with Different Molecular Weight Gelatins on Proanthocyanidin Composition and Perception of Wines. Am. J. Enol. Vitic. 2001, 52, 140–145. [Google Scholar]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of Commercial Mannoproteins on Wine Colour and Tannins Stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Pérez-Mestre, C.; Ferreras-Charro, R.; Rivero, F.J.; Heredia, F.J.; Escribano-Bailón, M.T. Addition of Mannoproteins and/or Seeds during Winemaking and Their Effects on Pigment Composition and Color Stability. J. Agric. Food Chem. 2019, 67, 4031–4042. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between Aroma Compounds and Whole Mannoprotein Isolated from Saccharomyces Cerevisiae Strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Jones, P.R.; Gawel, R.; Francis, I.L.; Waters, E.J. The Influence of Interactions between Major White Wine Components on the Aroma, Flavour and Texture of Model White Wine. Food Qual. Prefer. 2008, 19, 596–607. [Google Scholar] [CrossRef]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to Balance the Effect of Order of Presentation and First-Order Carry-over Effects in Hall Tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Glories, Y. La Couleur Des Vins Rouges. 1° e 2° Partie. Connaissance de la Vigne et du Vin 1984, 18, 253–271. [Google Scholar]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A New Simplified Method for Measuring the Colour of Wines. I. Red and rosé wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar]
- Di Stefano, R.; Guidoni, S. La Determinazione Dei Polifenoli Totali Nei Mosti e Nei Vini. Vignevini 1989, 16, 47–52. [Google Scholar]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of Polymeric Pigments in Grape Berry Extract Sand Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]



| Wine Typology | Samples | Total Anthocyanins mg/L | Colour Intensity (420 + 520 + 620) a.u. † | Hue | Polymeric Pigments (520) a.u. † | L* | a* | b* | ΔE |
|---|---|---|---|---|---|---|---|---|---|
| Extended Maceration (E) | E-C | 256.99 ± 0.84 a | 13.70 ± 0.02 a | 0.75 ± 0.00 b | 5.87 ± 0.07 c | 70.2 ± 0.2 c | 37.6 ± 0.3 a | 19.7 ± 0.3 a | - |
| E-MF | 252.13 ± 1.82 b | 13.05 ± 0.02 c | 0.75 ± 0.00 b | 6.06 ± 0.09 b | 71.5 ± 0.1 b | 36.6 ± 0.0 b | 19.3 ± 0.5 a | 1.79 b | |
| E-MP | 199.63 ± 0.44 d | 11.98 ± 0.06 d | 0.76 ± 0.01 a | 6.08 ± 0.02 b | 73.6 ± 0.1 a | 34.1 ± 0.3 c | 17.5 ± 0.6 b | 5.34 a | |
| E-MS | 228.97 ± 0.51 c | 13.21 ± 0.05 b | 0.75 ± 0.01 b | 6.28 ± 0.04 a | 71.4 ± 0.4 b | 37.1 ± 0.4 ab | 19.2 ± 0.3 a | 1.48 b | |
| Marc-Pressed (P) | P-C | 174.51 ± 2.86 c | 11.06 ± 0.47 ab | 0.72 ± 0.01 b | 3.77 ± 0.01 b | 68.2 ± 0.4 b | 28.8 ± 0.1 a | 11.2 ± 0.2 a | - |
| P-MF | 175.74 ± 0.28 bc | 10.76 ± 0.23 b | 0.72 ± 0.00 b | 4.02 ± 0.01 a | 66.7 ± 0.5 c | 29.2 ± 0.2 a | 11.2 ± 0.4 a | 1.58 b | |
| P-MP | 178.92 ± 2.30 b | 10.57 ± 0.16 b | 0.73 ± 0.00 b | 3.97 ± 0.09 ab | 70.2 ± 0.4 a | 26.8 ± 0.5 b | 10.4 ± 0.3 b | 2.98 a | |
| P-MS | 192.03 ± 1.57 a | 11.46 ± 0.13 a | 0.75 ± 0.01 a | 3.89 ± 0.20 ab | 66.7 ± 0.2 c | 29.0 ± 0.2 a | 10.9 ± 0.5 a | 1.58 b | |
| Free-Run (F) | F-C | 223.97 ± 4.98 | 12.63 ± 0.01 b | 0.68 ± 0.00 b | 4.42 ± 0.06 d | 62.6 ± 0.3 b | 49.3 ± 0.1 b | 25.8 ± 0.1 c | - |
| F-MF | 219.71 ± 19.70 | 13.10 ± 0.26 a | 0.70 ± 0.01 a | 4.70 ± 0.05 b | 62.8 ± 0.0 ab | 49.7 ± 0.0 a | 25.7 ± 0.1 c | 0.52 b | |
| F-MP | 207.78 ± 3.26 | 11.86 ± 0.01 c | 0.69 ± 0.00 ab | 4.54 ± 0.01 c | 63.1 ± 0.0 a | 49.4 ± 0.0 b | 26.6 ± 0.1 b | 0.91 b | |
| F-MS | 218.82 ± 6.96 | 12.88 ± 0.07 ab | 0.70 ± 0.00 a | 4.80 ± 0.06 a | 61.9 ± 0.2 c | 48.1 ± 0.2 c | 27.4 ± 0.2 a | 2.11 a |
| Extended Maceration Wines (E) | Marc-Pressed Wines (P) | Free-Run Wines (F) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | |
| Eigenvalue | 2.9 | 0.7 | 0.3 | 2.5 | 1.4 | 0.2 | 2.2 | 1.5 | 0.7 |
| Variability (%) | 75.9 | 17.5 | 6.6 | 61.2 | 35.0 | 3.7 | 50.8 | 34.2 | 15.0 |
| Cumulative % | 75.9 | 93.4 | 100.0 | 61.2 | 96.3 | 100.0 | 50.8 | 85.0 | 100.0 |
| Correlations | |||||||||
| Subquality | |||||||||
| Silk | 0.270 | 0.928 | 0.258 | −0.838 | 0.354 | 0.416 | 0.132 | −0.828 | 0.545 |
| Velvet | 0.984 | −0.178 | −0.010 | −0.962 | 0.220 | 0.161 | 0.493 | −0.868 | 0.054 |
| Dry | −0.860 | −0.471 | −0.197 | 0.710 | −0.703 | 0.051 | 0.101 | 0.623 | −0.776 |
| Corduroy | −0.064 | 0.834 | −0.548 | 0.942 | 0.330 | −0.058 | −0.713 | 0.695 | 0.092 |
| Adhesive | −0.942 | −0.136 | −0.308 | 0.464 | −0.883 | 0.077 | 0.525 | 0.087 | −0.846 |
| Hard | −0.753 | −0.600 | −0.269 | 0.772 | −0.495 | 0.398 | −0.367 | 0.819 | −0.441 |
| Aggressive | −0.996 | −0.088 | 0.013 | 0.867 | −0.444 | −0.226 | −0.140 | 0.990 | −0.033 |
| Soft | 0.917 | −0.398 | 0.009 | −0.823 | 0.551 | −0.138 | 0.795 | −0.587 | −0.153 |
| Mouthcoat | 0.991 | −0.097 | −0.097 | −0.869 | 0.491 | 0.060 | 0.143 | −0.919 | −0.368 |
| Rich | 0.439 | 0.389 | 0.810 | −0.280 | 0.785 | 0.552 | 0.959 | −0.192 | −0.206 |
| Green | −0.847 | −0.488 | −0.210 | 0.626 | −0.776 | 0.076 | −0.156 | 0.456 | −0.876 |
| Grainy | −0.948 | 0.102 | 0.302 | 0.765 | −0.428 | −0.482 | −0.686 | 0.727 | −0.038 |
| Satin | 0.970 | −0.245 | 0.001 | −0.393 | 0.795 | −0.462 | −0.994 | −0.055 | 0.096 |
| Pucker | −0.977 | −0.207 | −0.055 | 0.976 | 0.211 | −0.052 | −0.450 | 0.879 | −0.157 |
| Full-body | 0.365 | 0.812 | 0.455 | −0.826 | 0.506 | −0.250 | 0.679 | −0.342 | 0.650 |
| Persistent | 0.842 | 0.162 | 0.514 | −0.993 | 0.098 | −0.058 | −0.341 | −0.314 | 0.886 |
| Colour parameter | |||||||||
| L* | 0.997 | −0.072 | 0.028 | −0.776 | −0.522 | 0.353 | −0.853 | −0.507 | −0.122 |
| a* | −0.957 | 0.287 | −0.051 | −0.752 | −0.647 | −0.127 | −0.959 | −0.191 | 0.210 |
| b* | −0.961 | 0.232 | 0.147 | 0.378 | 0.846 | 0.376 | 0.941 | −0.270 | −0.202 |
| Hue | 0.935 | −0.330 | 0.128 | 0.304 | 0.952 | −0.046 | 0.971 | −0.134 | 0.199 |
| Colour Intensity | −0.988 | 0.146 | −0.060 | 0.899 | 0.348 | −0.267 | 0.177 | 0.670 | 0.721 |
| Polymeric pigments | 0.486 | 0.862 | −0.143 | −0.854 | 0.483 | −0.193 | 0.714 | −0.118 | 0.691 |
| Phenolic content | |||||||||
| Total Anthocyanins | −0.930 | 0.002 | 0.368 | 0.454 | 0.889 | 0.066 | −0.031 | 0.979 | 0.204 |
| BSA-reactive tannins | −0.923 | −0.342 | 0.176 | 0.976 | −0.179 | 0.121 | 0.952 | 0.296 | −0.076 |
| Vanillin-reactive flavans | −0.908 | 0.373 | −0.192 | −0.962 | 0.269 | 0.038 | −0.913 | −0.370 | 0.169 |
| Factor scores | |||||||||
| C | −2.006 | −0.729 | −0.215 | 1.486 | −1.660 | 0.038 | −1.106 | 1.611 | −0.836 |
| MF | −0.106 | 0.284 | 0.897 | −1.641 | −0.080 | −0.523 | −1.087 | 0.048 | 1.260 |
| MP | 2.768 | −0.702 | −0.169 | −1.467 | 0.242 | 0.573 | −0.469 | −1.882 | −0.615 |
| MS | 0.016 | 1.248 | −0.429 | 1.769 | 1.763 | −0.073 | 2.465 | 0.317 | 0.097 |
| Contributions (%) | |||||||||
| Subquality | 32.6 | 55.2 | 63.6 | 33.0 | 26.7 | 33.4 | 20.3 | 49.6 | 53.3 |
| Colour parameter | 33.7 | 30.1 | 5.2 | 27.6 | 43.7 | 60.3 | 43.2 | 13.5 | 41.3 |
| Phenolic content | 33.7 | 14.7 | 31.2 | 39.4 | 29.5 | 6.3 | 36.5 | 36.8 | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, A.; Gonzalez, A.; Moio, L.; Gambuti, A. Commercial Mannoproteins Improve the Mouthfeel and Colour of Wines Obtained by Excessive Tannin Extraction. Molecules 2021, 26, 4133. https://doi.org/10.3390/molecules26144133
Rinaldi A, Gonzalez A, Moio L, Gambuti A. Commercial Mannoproteins Improve the Mouthfeel and Colour of Wines Obtained by Excessive Tannin Extraction. Molecules. 2021; 26(14):4133. https://doi.org/10.3390/molecules26144133
Chicago/Turabian StyleRinaldi, Alessandra, Alliette Gonzalez, Luigi Moio, and Angelita Gambuti. 2021. "Commercial Mannoproteins Improve the Mouthfeel and Colour of Wines Obtained by Excessive Tannin Extraction" Molecules 26, no. 14: 4133. https://doi.org/10.3390/molecules26144133