Additive Effects of Lithium Salts with Various Anionic Species in Poly (Methyl Methacrylate)
Abstract
:1. Introduction
2. Results and Discussion
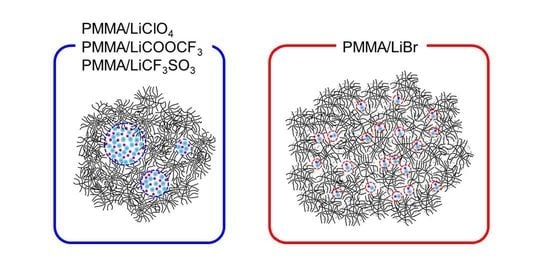
2.1. Effects of the Anion Characteristics of Salts
2.2. Effects of Anion Sizes of the Salts
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Characterization
3.3. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, J.; He, J.; Fan, D.; Tang, W.; Yang, Y. Thermal decomposition of dithioesters and its effect on RAFT polymerization. Macromolecules 2006, 39, 3753–3759. [Google Scholar] [CrossRef]
- Ma, J.; Lu, M.; Cao, C.; Zhang, H. Synthesis and characterization of PMMA/SiO2 organic-inorganic hybrid materials via RAFT-mediated miniemulsion polymerization. Polym. Compos. 2013, 34, 626–633. [Google Scholar] [CrossRef]
- Tan, W.L.; Bakar, M.A.; Bakar, N.H.H.A. Effect of anion of lithium salt on the property of lithium salt-epoxidized natural rubber polymer electrolytes. Ionics 2013, 19, 601–613. [Google Scholar] [CrossRef]
- Hayashi, M.; Kimura, T.; Oba, Y.; Takasu, A. One-Pot Synthesis of Dual supramolecular associative PMMA-based copolymers and the precise thermal property tuning. Macromol. Chem. Phys. 2021, 222, 2000302. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Longworth, R.; Vaughan, D.J. Physical Structure of Ionomers. Nature 1968, 218, 85–87. [Google Scholar] [CrossRef]
- Kim, J.; Jackman, R.J.; Eisenberg, A. Filler and percolation behavior of ionic aggregates in styrene-sodium methacrylate ionomers. Macromolecules 1994, 27, 2789–2803. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly (ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Armand, M. Polymer solid electrolytes-an overview. Solid State Ionics 1983, 9–10, 745–754. [Google Scholar] [CrossRef]
- Tominaga, Y.; Izumi, Y.; Kwak, G.H.; Asai, S.; Sumita, M. Effect of supercritical carbon dioxide processing on ionic association and conduction in a crystalline poly(ethylene oxide)-LiCF3SO3 complex. Macromolecules 2003, 36, 8766–8772. [Google Scholar] [CrossRef]
- Tominaga, Y.; Asai, S.; Sumita, M. Relation between ionic conductivity and solubility of CO2 in pressurized solid polymer electrolytes. Macromolecules 2007, 40, 3348–3354. [Google Scholar] [CrossRef]
- Agnihotry, S.A.; Pradeep, P.; Sekhon, S.S. PMMA based gel electrolyte for EC smart windows. Electrochim. Acta 1999, 44, 3121–3126. [Google Scholar] [CrossRef]
- Shukla, N.; Thakur, A.K. Role of salt concentration on conductivity optimization and structural phase separation in a solid polymer electrolyte based on PMMA-LiClO4. Ionics 2009, 15, 357–367. [Google Scholar] [CrossRef]
- TianKhoon, L.; Ataollahi, N.; Hassan, N.H.; Ahmad, A. Studies of porous solid polymeric electrolytes based on poly (vinylidene fluoride) and poly (methyl methacrylate) grafted natural rubber for applications in electrochemical devices. J. Solid State Electrochem. 2016, 20, 203–213. [Google Scholar] [CrossRef]
- Kurapati, S.; Gunturi, S.S.; Nadella, K.J.; Erothu, H. Novel solid polymer electrolyte based on PMMA:CH3COOLi effect of salt concentration on optical and conductivity studies. Polym. Bull. 2019, 76, 5463–5481. [Google Scholar] [CrossRef]
- Ito, A.; Ayerdurai, V.; Miyagawa, A.; Matsumoto, A.; Okada, H.; Courtoux, A.; Yamaguchi, M. Effects of residual solvent on glass transition temperature of poly(methyl methacrylate). Nihon Reoroji Gakkaishi 2018, 46, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Maeno, R.; Yamaguchi, M. Control of optical and mechanical properties of poly(methyl methacrylate) by introducing lithium salt. Opt. Mater. 2018, 83, 152–156. [Google Scholar] [CrossRef]
- Ito, A.; Phulkerd, P.; Ayerdurai, V.; Soga, M.; Courtoux, A.; Miyagawa, A.; Yamaguchi, M. Enhancement of the glass transition temperature of poly(methyl methacrylate) by salt. Polym. J. 2018, 50, 857–863. [Google Scholar] [CrossRef]
- Miyagawa, A.; Ayerdurai, V.; Nobukawa, S.; Yamaguchi, M. Viscoelastic properties of poly(methyl methacrylate) with high glass transition temperature by lithium salt addition. J. Polym. Sci. Part. B Polym. Phys. 2016, 54, 2388–2394. [Google Scholar] [CrossRef]
- Stancik, A.L.; Brauns, E.B. A simple asymmetric lineshape for fitting infrared absorption spectra. Vib. Spectrosc. 2008, 47, 66–69. [Google Scholar] [CrossRef]
- Wieczorek, W.; Lipka, P.; Żukowska, G.; Wyciślik, H. Ionic interactions in polymeric electrolytes based on low molecular weight poly(ethylene glycol)s. J. Phys. Chem. B 1998, 102, 6968–6974. [Google Scholar] [CrossRef]
- Ito, A. Effect of Addition of Lithium Salts on Properties of Poly(Methyl Methacrylate). Ph.D. Thesis, Japan Advanced Institute of Science and Technology (JAIST), Nomi, Japan, 2019. [Google Scholar]
- Regis, A.; Corset, J. IR study of solutions of lithium trifluoroacetate in non-aqueous solvents. Structure of ion pairs and triple ions. Chem. Phys. Lett. 1975, 32, 462–465. [Google Scholar] [CrossRef]
- Frech, R.; Huang, W. Polymer conformation and ionic association in complexes of lithium, sodium and potassium triflate with poly (ethylene oxide) oligomers. Solid State Ionics 1994, 72, 103–107. [Google Scholar] [CrossRef]
- Pásztor, S.; Becsei, B.; Szarka, G.; Thomann, Y.; Thomann, R.; Mühlhaupt, R.; Iván, B. The scissors effect in action: The fox-flory relationship between the glass transition temperature of crosslinked poly(methyl methacrylate) and Mc in nanophase separated poly(methyl methacrylate)-l-polyisobutylene conetworks. Materials 2020, 13, 4822. [Google Scholar] [CrossRef]
- Willis, H.A.; Zichy, V.J.I.; Hendra, P.J. The laser-Raman and infra-red spectra of poly(methyl methacrylate). Polymer 1969, 10, 737–746. [Google Scholar] [CrossRef]
- Brinkhuis, R.H.G.; Schouten, A.J. Thin-film behavior of poly(methyl methacrylates). 2. An FT-IR study of Langmuir-Blodgett films of isotactic PMMA. Macromolecules 1991, 24, 1496–1504. [Google Scholar] [CrossRef]
- Havriliak, S.; Roman, N. The infra-red absorption characteristics of syndiotactic poly(methyl methacrylate) from 1050 cm−1 to 1300 cm−1. Polymer 1966, 7, 387–400. [Google Scholar] [CrossRef]








| Sample Code | Tg/°C |
|---|---|
| PMMA | 127 |
| PMMA/LiClO4 | 126 |
| PMMA/LiCOOCF3 | 133 |
| PMMA/LiCF3SO3 | 137 |
| PMMA/LiBr | 133 |
| Sample Code | Peak Area/% | ||
|---|---|---|---|
| PMMA/LiClO4 | PMMA/LiCOOCF3 | PMMA/LiCF3SO3 | |
| Free | 72.3 | 44.1 | 17.3 |
| Ion pair | 27.7 | 40.8 | 42.7 |
| Triple ion | ― | 5.7 | 40.0 |
| Aggregates | ― | 9.3 | ― |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, A.; Nitta, K.-h. Additive Effects of Lithium Salts with Various Anionic Species in Poly (Methyl Methacrylate). Molecules 2021, 26, 4096. https://doi.org/10.3390/molecules26134096
Ito A, Nitta K-h. Additive Effects of Lithium Salts with Various Anionic Species in Poly (Methyl Methacrylate). Molecules. 2021; 26(13):4096. https://doi.org/10.3390/molecules26134096
Chicago/Turabian StyleIto, Asae, and Koh-hei Nitta. 2021. "Additive Effects of Lithium Salts with Various Anionic Species in Poly (Methyl Methacrylate)" Molecules 26, no. 13: 4096. https://doi.org/10.3390/molecules26134096