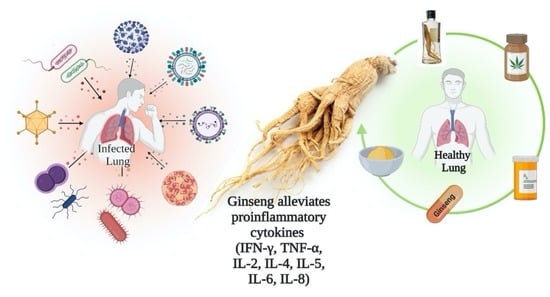
Pharmacological Efficacy of Ginseng against Respiratory Tract Infections
Abstract
:1. Introduction
2. The Methodology of the Literature Review
3. Ginseng Structural Features
4. Pathogenicity of Microbial Infections
5. Ginseng’s Immunomodulator Effect
6. Impact of Ginseng on Respiratory Virus Infections
6.1. Influenza Virus
6.2. Respiratory Syncytial Virus
6.3. Rhinoviruses
6.4. Coronaviruses
7. Anti-Bacterial Activity of Ginseng
7.1. Pseudomonas Aeruginosa
7.2. Streptococcus Pneumonia
8. Ginseng Clinical Trials
Evaluations of Ginseng’s Impact on Respiratory Pathogens in Human Clinical Trials
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Technical Report Series 954 Evaluation of Certain Veterinary Drug Residues in Food Seventieth Report of the Joint FAO/WHO Expert Committee on Food Additives Food and Agriculture Organization of the United Nations World Health Organization; WHO: Geneva, Switzerland, 2009; ISBN 9789241209540. [Google Scholar]
- Martino, D.; Prescott, S. Epigenetics and prenatal influences on asthma and allergic airways disease. Chest 2011, 139, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Brugha, R.; Grigg, J. Urban air pollution and respiratory infections. Paediatr. Respir. Rev. 2014, 15, 194–199. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Emerging anti-inflammatory strategies for COPD. Eur. Respir. J. 2012, 40, 724–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Ishihara, Y.; Takemoto, T.; Ishida, A.; Yamazaki, T. Protective actions of 17 β -Estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds. Oxid. Med. Cell. Longev. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.C.; Shergis, J.L.; Zhang, A.L.; Worsnop, C.; Fong, H.; Story, D.; Da Costa, C.; Thien, F.C.K. Panax ginseng C.A Meyer root extract for moderate Chronic Obstructive Pulmonary Disease (COPD): Study protocol for a randomised controlled trial. Trials 2011, 12, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilia, A.R. Science meets regulation. J. Ethnopharmacol. 2014, 158, 487–494. [Google Scholar] [CrossRef]
- Blumenthal, M. The ABC Clinical Guide to Herbs; American Botanical Council: Austin, TX, USA, 2003. [Google Scholar]
- Jia, L.; Zhao, Y.; Liang, X.-J. Current Evaluation of the Millennium Phytomedicine- Ginseng (II): Collected Chemical Entities, Modern Pharmacology, and Clinical Applications Emanated from Traditional Chinese Medicine. Curr. Med. Chem. 2009, 16, 2924–2942. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- An, X.; Zhang, A.L.; Yang, A.W.; Lin, L.; Wu, D.; Guo, X.; Shergis, J.L.; Thien, F.C.K.; Worsnop, C.J.; Xue, C.C. Oral ginseng formulae for stable chronic obstructive pulmonary disease: A systematic review. Respir. Med. 2011, 105, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Gross, D.; Krieger, D.; Efrat, R.; Dayan, M. Ginseng extract G115® for the treatment of chronic respiratory diseases. Scweiz. Z. Ganzheits. Med. 1995, 1, 29–33. [Google Scholar]
- Scaglione, F.; Weiser, K.; Alessandria, M. Effects of the standardised ginseng extract G115® in patients with chronic bronchitis: A nonblinded, randomised, comparative pilot study. Clin. Drug Investig. 2001, 21, 41–45. [Google Scholar] [CrossRef]
- Coon, J.T.; Ernst, E. Panax ginseng. Drug Saf. 2002, 25, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tanaka, O.; Ishii, T.; Fujita, M.; Itokawa, H. Studies on the Constituents of Japanese and Chinese Crude Drugs. XI.∗ Panaxadiol, A Sapogenin of Ginseng Roots. (2). Chem. Pharm. Bull. 1963, 11, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, W.S.; Lee, D.G. In Vitro Candidacidal Action of Korean Red Ginseng Saponins against Candida albicans. Biol. Pharm. Bull. 2008, 31, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Mallavadhani, U.V.; Mahapatra, A.; Raja, S.S.; Manjula, C. Antifeedant activity of some pentacyclic triterpene acids and their fatty acid ester analogues. J. Agric. Food Chem. 2003, 51, 1952–1955. [Google Scholar] [CrossRef]
- Katerere, D.R.; Gray, A.I.; Nash, R.J.; Waigh, R.D. Antimicrobial activity of pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry 2003, 63, 81–88. [Google Scholar] [CrossRef]
- Ginseng: Nature’s Anti-Inflammatory?—ScienceDaily. Available online: https://www.sciencedaily.com/releases/2009/05/090513215410.htm (accessed on 1 December 2020).
- Lee, D.C.W.; Yang, C.L.H.; Chik, S.C.C.; Li, J.C.B.; Rong, J.H.; Chan, G.C.F.; Lau, A.S.Y. Bioactivity-guided identification and cell signaling technology to delineate the immunomodulatory effects of Panax ginseng on human promonocytic U937 cells. J. Transl. Med. 2009, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Lee, Y.N.; Lee, Y.-T.; Hwang, H.S.; Kim, K.-H.H.; Ko, E.-J.J.; Kim, M.-C.C.; Kang, S.-M.M. Ginseng Protects Against Respiratory Syncytial Virus by Modulating Multiple Immune Cells and Inhibiting Viral Replication. Nutrients 2015, 7, 1021–1036. [Google Scholar] [CrossRef]
- Silvestrini, P.; Beccaria, C.; Pereyra, E.A.L.; Renna, M.S.; Ortega, H.H.; Calvinho, L.F.; Dallard, B.E.; Baravalle, C. Intramammary inoculation of Panax ginseng plays an immunoprotective role in Staphylococcus aureus infection in a murine model. Res. Vet. Sci. 2017, 115, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Sun, H.; Wang, S.; Guo, X.; Ding, H.; Yang, Y.; Shan, Y.; Du, A. Ginseng stem-and-leaf saponin (GSLS)-Enhanced protective immune responses induced by Toxoplasma gondii heat shocked protein 70 (HSP70) against toxoplasmosis in mice. J. Parasitol. 2017, 103, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Rhee, D. kwon Ginseng alleviates microbial infections of the respiratory tract: A review. J. Ginseng Res. 2020, 44, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Jiang, J.; Jamaluddin, M.S.; Liang, Z.; Yao, Q.; Chen, C. Ginsenoside Rb1 blocks ritonavir-induced oxidative stress and eNOS downregulation through activation of estrogen receptor-beta and upregulation of SOD in human endothelial cells. Int. J. Mol. Sci. 2019, 20, 294. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef]
- Chang-Xiao, L.; Pei-Gen, X. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992, 36, 27–38. [Google Scholar] [CrossRef]
- Yang, M.S.; Wu, M.Y. Chinese Ginseng. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 693–705. ISBN 9780128021477. [Google Scholar]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [Green Version]
- CHENG, Y.; SHEN, L.; ZHANG, J. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med. Sci. Monit. 2004, 10, RA187–RA192. [Google Scholar]
- Lim, K.H.; Lim, D.J.; Kim, J.H. Ginsenoside-Re ameliorates ischemia and reperfusion injury in the heart: A hemodynamics approach. J. Ginseng Res. 2013, 37, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, C.; Yeh, G.Y.; Phillips, R.S.; Mittleman, M.A.; Kaptchuk, T.J. Systematic review of the of ginseng on cardiovascular risk factors. Ann. Pharmacother. 2006, 40, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, C.T. Pharmacological effects of ginseng on infectious diseases. Inflammopharmacology 2019, 27, 871–883. [Google Scholar] [CrossRef]
- Fonkwo, P.N. Pricing infectious disease. EMBO Rep. 2008, 9, S13–S17. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.D.; Gray, G.C. Emerging and reemerging infectious diseases. In Encyclopedia of Microbiology; Nature Publishing Group: Berlin, Germany, 2019; pp. 112–122. ISBN 9780128117378. [Google Scholar]
- Bakaletz, L.O. Viral–bacterial co-infections in the respiratory tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, G.; Dunn, G.; Chadwick, P.; Blackwood, B.; McAuley, D.; Perkins, G.D.; McMullan, R.; Gates, S.; Bentley, A.; Young, D.; et al. Rapid detection of health-care-associated bloodstream infection in critical care using Multipathogen real-time polymerase chain reaction technology: A diagnostic accuracy study and systematic review. Health Technol. Assess. 2015, 19, 1–141. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.G.; Balm, M.N.D.; Blackmore, T.K. Molecular testing for viral and bacterial enteric pathogens: Gold standard for viruses, but don’t let culture go just yet? Pathology 2015, 47, 227–233. [Google Scholar] [CrossRef]
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef]
- Yu, C.; Wang, C.Z.; Zhou, C.J.; Wang, B.; Han, L.; Zhang, C.F.; Wu, X.H.; Yuan, C.S. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J. Pharm. Biomed. Anal. 2014, 99, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yu, Q.-T.; Ge, Y.-Z.; Zhang, W.-S.; Fan, Y.; Ma, C.-W.; Liu, Q.; Qi, L.-W. Distinct urine metabolome after Asian ginseng and American ginseng intervention based on GC-MS metabolomics approach. Sci. Rep. 2016, 6, 39045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, O.R.; Nguyen, N.Q.; Lee, K.H.; Kim, Y.C.; Seo, J. Cytohistological study of the leaf structures of Panax ginseng Meyer and Panax quinquefolius L. J. Ginseng Res. 2017, 41, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Wang, X.; Sun, G.; Li, F.; Gong, X. Application of RAD sequencing for evaluating the genetic diversity of domesticated Panax notoginseng (Araliaceae). PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchers, A.T.; Keen, C.L.; Stern, J.S.; Gershwin, M.E. Inflammation and Native American medicine: The role of botanicals. Am. J. Clin. Nutr. 2000, 72, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Lee, R.; Nam, S.M.; Kim, D.-G.; Cho, I.-H.; Kim, H.-C.; Cho, Y.; Rhim, H.; Nah, S.-Y. Ginseng gintonin, aging societies, and geriatric brain diseases. Integr. Med. Res. 2021, 10, 100450. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef] [Green Version]
- Straughn, A.R.; Kakar, S.S. Withaferin A: A potential therapeutic agent against COVID-19 infection. J. Ovarian Res. 2020, 13, 1–5. [Google Scholar] [CrossRef]
- Gaurav, N. A Review: Pharmacological and Medicinal Activity of Active Compounds of Withania somnifera. J. Eng. Technol. 2013, 3, 2231. [Google Scholar]
- Tiwari, R.; Chakraborty, S.; Saminathan, M.; Dhama, K.; Singh, S.V. Ashwagandha (Withania somnifera): Role in safeguarding health, immunomodulatory effects, combating infections and therapeutic applications: A Review. J. Biol. Sci. 2014, 14, 77–94. [Google Scholar] [CrossRef] [Green Version]
- Shergis, J.L.; Di, Y.M.; Zhang, A.L.; Vlahos, R.; Helliwell, R.; Ye, J.M.; Xue, C.C. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement. Med. 2014, 22, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Scaglione, F.; Ferrara, F.; Dugnani, S.; Falchi, M.; Santoro, G.; Fraschini, F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp. Clin. Res. 1990, 16, 537–542. [Google Scholar]
- Cui, L.; Wu, S.Q.; Zhao, C.A.; Yin, C.R. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. J. Ginseng Res. 2016, 40, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.T. Botanical Characteristics, Pharmacological Effects and Medicinal Components of Korean Panax ginseng C A Meyer; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; Volume 29, pp. 1109–1118. [Google Scholar]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. 2004, 9, 259–274. [Google Scholar] [PubMed]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Hu, S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine 2009, 27, 4883–4890. [Google Scholar] [CrossRef]
- Quan, F.S.; Compans, R.W.; Cho, Y.K.; Kang, S.M. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 2007, 25, 272–282. [Google Scholar] [CrossRef]
- Lee, J.I.; Park, K.S.; Cho, I.H. Panax ginseng: A candidate herbal medicine for autoimmune disease. J. Ginseng Res. 2019, 43, 342–348. [Google Scholar] [CrossRef]
- Kim, C.J.; Ryu, H.Y.; Lee, S.; Lee, H.J.; Chun, Y.S.; Kim, J.K.; Yu, C.Y.; Ghimire, B.K.; Lee, J.G. Neuroprotective effect and antioxidant potency of fermented cultured wild ginseng root extracts of Panax ginseng C.A. meyer in mice. Molecules 2021, 26, 3001. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Yang, A.Y.; Cheng, D.C.; Boyanapalli, S.S.S.; Su, Z.Y.; Khor, T.O.; Gao, S.; Wang, J.; Jiang, Z.H.; Kong, A.N.T. Pharmacodynamics of ginsenosides: Antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem. Res. Toxicol. 2012, 25, 1574–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.-M.; Yao, Q.; Chen, C. Ginseng Compounds: An Update on Their Molecular Mechanisms and Medical Applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.; Lemon, S.M. Hepatitis A virus: From discovery to vaccines. Hepatology 2006, 43, S164–S172. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, J.G.; Park, S.Y.; Bae, O.N.; Kim, D.H.; Park, J.H. Pectic polysaccharides from Panax ginseng as the antirotavirus principals in ginseng. Biomacromolecules 2010, 11, 2044–2052. [Google Scholar] [CrossRef]
- Song, X.; Chen, J.; Sakwiwatkul, K.; Li, R.; Hu, S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int. Immunopharmacol. 2010, 10, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M. Respiratory syncytial virus in infants. Curr. Opin. Infect. Dis. 2015, 28, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Porter, K. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet 2003, 362, 1267–1274. [Google Scholar] [CrossRef]
- Munos, M.K.; Fischer Walker, C.L.; Black, R.E. The effect of rotavirus vaccine on diarrhoea mortality. Int. J. Epidemiol. 2010, 39, i56–i62. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.L.; Lind, A.; Margolis, K.L.; Murdoch, M.; McFadden, R.; Hauge, M.; Magnan, S.; Drake, M. The Effectiveness of Vaccination against Influenza in Healthy, Working Adults. N. Engl. J. Med. 1995, 333, 889–893. [Google Scholar] [CrossRef]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: Outbreaks and biological properties. Cell Res. 2010, 20, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Claas, E.C.J.; Osterhaus, A.D.M.E.; Van Beek, R.; De Jong, J.C.; Rimmelzwaan, G.F.; Senne, D.A.; Krauss, S.; Shortridge, K.F.; Webster, R.G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998, 351, 472–477. [Google Scholar] [CrossRef]
- Dong, W.; Farooqui, A.; Leon, A.J.; Kelvin, D.J. Inhibition of influenza A virus infection by ginsenosides. PLoS ONE 2017, 12, e0171936. [Google Scholar] [CrossRef]
- Wang, Y.; Jung, Y.-J.J.; Kim, K.-H.H.; Kwon, Y.; Kim, Y.-J.J.; Zhang, Z.; Kang, H.-S.S.; Wang, B.-Z.Z.; Quan, F.-S.S.; Kang, S.-M.M. Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses. Viruses 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, G.M.; Arroll, B. Prevention and treatment of the common cold: Making sense of the evidence. Can. Med. Assoc. J. 2014, 186, 190–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaglione, F.; Cattaneo, G.; Alessandria, M.; Cogo, R. Efficacy and safety of the standardized ginseng extract G 115 for potentiating vaccination against common cold and/or influenza syndrome. Drugs Exp. Clin. Res. 1996, 22, 65–72. [Google Scholar] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Traditional Herbal Medicine for the Prevention and Treatment of Cold and Flu in the Autumn of 2020, Overlapped With COVID-19. Nat. Prod. Commun. 2020, 15, 1934578X2095143. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Li, Z.; Ma, X.; Cui, X.; Chi, X.; Xu, W.; Hu, S. Sunflower seed oil containing ginseng stem–leaf saponins (E515-D) is a safe adjuvant for Newcastle disease vaccine. Poult. Sci. 2020, 99, 4795–4803. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chi, X.; Yuan, L.; Wang, Y.; Li, Z.; Xu, W.; Rajput, Z.I.; Hu, S. Immunomodulatory effect of ginseng stem-leaf saponins and selenium on Harderian gland in immunization of chickens to Newcastle disease vaccine. Vet. Immunol. Immunopathol. 2020, 225, 110061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, C.; He, Z.; Wei, C.; Su, N. Literature Analysis of the Efficacy of Arbidol in Virus Infectious Diseases. Biointerface Res. Appl. Chem. 2020, 11, 7646–7658. [Google Scholar] [CrossRef]
- Sarkar, I.; Zardini Buzatto, A.; Garg, R.; Li, L.; Van Drunen Littel-Van Den Hurk, S. Metabolomic and Immunological Profiling of Respiratory Syncytial Virus Infection after Intranasal Immunization with a Subunit Vaccine Candidate. J. Proteome Res. 2019, 18, 1145–1161. [Google Scholar] [CrossRef]
- Xing, Y.; Proesmans, M. New therapies for acute RSV infections: Where are we? Eur. J. Pediatr. 2019, 178, 131–138. [Google Scholar] [CrossRef]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Friedland, J.S.; Remick, D.G.; Davies, E.G.; Sharland, M. Elevated Plasma Interleukin 8 in Respiratory Syncytial Virus Bronchiolitis. Pediatr. Infect. Dis. J. 1995, 14, 919. [Google Scholar] [CrossRef]
- Lee, J.S.; Hwang, H.S.; Ko, E.J.; Lee, Y.N.; Kwon, Y.M.; Kim, M.C.; Kang, S.M. Immunomodulatory activity of red ginseng against influenza a virus infection. Nutrients 2014, 6, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, J.C.G.; Richardson, J.Y.; Darnell, M.E.R.; Rowzee, A.; Pletneva, L.; Porter, D.D.; Prince, G.A. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J. Infect. Dis. 2002, 185, 1780–1785. [Google Scholar] [CrossRef]
- Zhang, Y.; Luxon, B.A.; Casola, A.; Garofalo, R.P.; Jamaluddin, M.; Brasier, A.R. Expression of Respiratory Syncytial Virus-Induced Chemokine Gene Networks in Lower Airway Epithelial Cells Revealed by cDNA Microarrays. J. Virol. 2001, 75, 9044–9058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, D.F.; Yu, H.J.; Liu, Z.; Zhang, D.F.; Zhou, Q.J.; Zhang, H.L.; Du, A.F. Ginsenoside Rg1 enhances immune response induced by recombinant Toxoplasma gondii SAG1 antigen. Vet. Parasitol. 2011, 179, 28–34. [Google Scholar] [CrossRef]
- Li, L.C.; Piao, H.M.; Zheng, M.Y.; Lin, Z.H.; Choi, Y.H.; Yan, G.H. Ginsenoside Rh2 attenuates allergic airway inflammation by modulating nuclear factor-κB activation in a murine model of asthma. Mol. Med. Rep. 2015, 12, 6946–6954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.M.; Jung, H.J.; Choi, W.Y.; Lim, C.J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol. Biol. Rep. 2013, 40, 269–279. [Google Scholar] [CrossRef]
- Jung, I.D.; Kim, H.Y.; Park, J.W.; Lee, C.M.; Noh, K.T.; Kang, H.K.; Heo, D.R.; Lee, S.J.; Son, K.H.; Park, H.J.; et al. RG-II from Panax ginseng C.A. Meyer suppresses asthmatic reaction. BMB Rep. 2012, 45, 79–84. [Google Scholar] [CrossRef] [Green Version]
- van der Heide, S.L.; Xi, Y.; Upham, J.W. Natural Killer Cells and Host Defense Against Human Rhinoviruses Is Partially Dependent on Type I IFN Signaling. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Zhu, J.; Message, S.D.; Qiu, Y.; Mallia, P.; Kebadze, T.; Contoli, M.; Ward, C.K.; Barnathan, E.S.; Mascelli, M.A.; Kon, O.M.; et al. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest 2014, 145, 1219–1229. [Google Scholar] [CrossRef] [Green Version]
- Im, K.; Kim, J.; Min, H. Ginseng, the natural effectual antiviral: Protective effects of Korean red ginseng against viral infection. J. Ginseng Res. 2016, 40, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Arden, K.E.; McErlean, P.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J. Med. Virol. 2006, 78, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; An, J.M.; Jung, J.H.; Kang, I.G.; Choi, Y.S.; Kim, S.T. Korean red ginseng attenuates rhinovirus-stimulated IL-8, IL-6 responses in human nasal epithelial cells. Int. J. Clin. Exp. Med. 2017, 10, 1567–1574. [Google Scholar]
- Wahab, S.; Ahmad, I.; Irfan, S.; Baig, M.H.; Farouk, A.-E.; Dong, J.-J. Use of Natural Compounds as a Potential Therapeutic Agent Against COVID-19. Curr. Pharm. Des. 2021, 27, 1144–1152. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, M.F.; Hussain, A.; Usmani, S.; Shoaib, A.; Ahmad, W. Effectiveness of Azithromycin as add-on Therapy in COVID-19 Management. Mini-Rev. Med. Chem. 2021, 21. [Google Scholar] [CrossRef]
- Wahab, S.; Ahmad, I.; Usmani, S.; Ahmad, M.P. Epidemiological Situation and Efficacy of Dexamethasone for the treatment planning of COVID-19: A perspective review. Curr. Drug Deliv. 2020, 17. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Zhang, W.; Wu, X.; Huang, T.; Li, H.; Zheng, Y.; Che, J.; Sun, S.; Jiang, C.; Zhou, S.; et al. Shenhuang granule in the treatment of severe coronavirus disease 2019 (COVID-19): Study protocol for an open-label randomized controlled clinical trial. Trials 2020, 21, 568. [Google Scholar] [CrossRef] [PubMed]
- Jalali, A.; Dabaghian, F.; Akbrialiabad, H.; Foroughinia, F.; Zarshenas, M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID -19. Phyther. Res. 2020, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Leung, F.P.; Mak, N.K.; Tombran-Tink, J.; Huang, Y.; Wong, R.N. Protopanaxadiol and protopanaxatriol bind to glucocorticoid and oestrogen receptors in endothelial cells. Br. J. Pharmcol. 2009, 156, 626–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, W.-J.; Gu, C.-J.; Wu, K.; Yang, H.-L.; Mei, J.; Yu, J.-J.; Hou, X.-F.; Sun, J.-S.; Xu, F.-Y.; et al. The ginsenoside PPD exerts anti-endometriosis effects by suppressing estrogen receptor-mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell Death Dis. 2018, 9, 574. [Google Scholar] [CrossRef]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohé, C.; Laufs, K.; Böhm, M.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American ginseng (Panax quinquefolium L.) as a source of bioactive phytochemicals with pro-health properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef] [Green Version]
- McElhaney, J.E.; Gravenstein, S.; Cole, S.K.; Davidson, E.; O’Neill, D.; Petitjean, S.; Rumble, B.; Shan, J.J. A Placebo-Controlled Trial of a Proprietary Extract of North American Ginseng (CVT-E002) to Prevent Acute Respiratory Illness in Institutionalized Older Adults. J. Am. Geriatr. Soc. 2004, 52, 13–19. [Google Scholar] [CrossRef]
- Predy, G.N.; Goel, V.; Lovlin, R.; Donner, A.; Stitt, L.; Basu, T.K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: A randomized controlled trial. CMAJ 2005, 173, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElhaney, J.E.; Goel, V.; Toane, B.; Hooten, J.; Shan, J.J. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: A randomized, double-blinded, placebo controlled trial. J. Altern. Complement. Med. 2006, 12, 153–157. [Google Scholar] [CrossRef]
- Zhou, P.; Lu, S.; Luo, Y.; Wang, S.; Yang, K.; Zhai, Y.; Sun, G.; Sun, X. Attenuation of TNF-α-induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-κB, JNK and p38 signaling pathways. Front. Pharmcol. 2017, 8, 464. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.D.; Coimbra, C.C.; Marubayashi, U. Tryptophan-induced central fatigue in exercising rats is related to serotonin content in preoptic area. Neurosci. Lett. 2007, 415, 274–278. [Google Scholar] [CrossRef]
- Yang, X.; Lv, Y.; Tian, L.; Zhao, Y. Composition and systemic immune activity of the polysaccharides from an herbal tea (lycopus lucidus turcz). J. Agric. Food Chem. 2010, 58, 6075–6080. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, J.H.; Oh, M.; Choi, K.M.; Jeong, M.R.; Park, J.D.; Kwon, D.Y.; Ha, K.C.; Park, E.O.; Lee, N.; et al. Preventive effect of korean red ginseng for acute respiratory illness: A randomized and double-blind clinical trial. J. Korean Med. Sci. 2012, 27, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Rhee, D.K. Corona-Cov-2 (COVID-19) and ginseng: Comparison of possible use in COVID-19 and influenza. J. Ginseng Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; POKHREL, S.; Singh, J.; Varshney, A. Withanone from Withania somnifera May Inhibit Novel Coronavirus (COVID-19) Entry by Disrupting Interactions between Viral S-Protein Receptor Binding Domain and Host ACE2 Receptor. Res. Sq. 2020. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, M.K.; Singh, P.; Sharma, S.; Singh, T.P.; Ethayathulla, A.S.; Kaur, P. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn. 2020, 1–14. [Google Scholar] [CrossRef]
- Lu, C.C.; Chen, M.Y.; Lee, W.S.; Chang, Y.L. Potential therapeutic agents against COVID-19: What we know so far. J. Chin. Med. Assoc. 2020, 83, 534–536. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef]
- Tandon, N.; Yadav, S.S. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol. 2020, 255, 112768. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Namdeo, A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar] [CrossRef] [PubMed]
- Ashwagandha the New HCQ? Modi Govt Begins Study to See If Herb Keeps Coronavirus Away. Available online: https://theprint.in/health/ashwagandha-the-new-hcq-modi-govt-begins-study-to-see-if-herb-keeps-coronavirus-away/421830/ (accessed on 28 June 2021).
- Saggam, A.; Limgaokar, K.; Borse, S.; Chavan-Gautam, P.; Dixit, S.; Tillu, G.; Patwardhan, B. Withania somnifera (L.) Dunal: Opportunity for Clinical Repurposing in COVID-19 Management. Front. Pharmcol. 2021, 12, 835. [Google Scholar]
- Fallah, M.S.; Bayati, M.; Najafi, A.; Behmard, E.; Davarpanah, S.J. Molecular docking investigation of antiviral herbal compounds as potential inhibitors of sars-cov-2 spike receptor. Biointerface Res. Appl. Chem. 2021, 11, 12916–12924. [Google Scholar] [CrossRef]
- Chikhale, R.V.; Gurav, S.S.; Patil, R.B.; Sinha, S.K.; Prasad, S.K.; Shakya, A.; Shrivastava, S.K.; Gurav, N.S.; Prasad, R.S. Sars-cov-2 host entry and replication inhibitors from Indian ginseng: An in-silico approach. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, B.; Yu, D.; Olanrewaju, A.A.; Chilin, L.D.; He, S.; Dabbagh, D.; Alluhaibi, G.; Ma, Y.-C.; Hofmann, L.A.; Hakami, R.M.; et al. A traditional medicine, respiratory detox shot (RDS), inhibits the infection of SARS-CoV, SARS-CoV-2, and the influenza A virus in vitro. Cell Biosci. 2021, 11, 100. [Google Scholar] [CrossRef]
- Zhai, L.; Li, Y.; Wang, W.; Wang, Y.; Hu, S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine 2011, 29, 5007–5014. [Google Scholar] [CrossRef]
- Yin, S.Y.; Kim, H.J.H.J.; Kim, H.J.H.J. A comparative study of the effects of whole red ginseng extract and polysaccharide and saponin fractions on influenza A (H1N1) virus infection. Biol. Pharm. Bull. 2013, 36, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Song, J.M.; Quan, F.S.; Park, K.M.; Cho, Y.K.; Kang, S.M. Protective effect of korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J. Med. Food 2012, 15, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.-G.G.; Kim, M.-C.C.; Park, M.-K.K.; Park, K.-M.M.; Quan, F.-S.S.; Song, J.-M.M.; Wee, J.J.; Wang, B.-Z.Z.; Cho, Y.-K.K.; Compans, R.W.; et al. Protective Effect of Ginseng Polysaccharides on Influenza Viral Infection. PLoS ONE 2012, 7, e33678. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, S.W.; Park, S.J.; Kim, S.; Yu, K.M.; Kim, S.G.; Lee, S.H.; Seo, Y.K.; Cho, N.H.; Kang, K.; et al. Greater efficacy of black ginseng (CJ enerG) over red ginseng against lethal influenza a virus infection. Nutrients 2019, 11, 1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Cho, M.K.; Hwang, H.S.; Ko, E.-J.; Lee, Y.-N.; Kwon, Y.-M.; Kim, M.-C.; Kim, K.-H.; Lee, Y.-T.; Jung, Y.-J.; et al. Ginseng diminishes lung disease in mice immunized with formalin-inactivated respiratory syncytial virus after challenge by modulating host immune responses. J. Interferon Cytokine Res. 2014, 34, 902–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Ko, E.J.; Hwang, H.S.; Lee, Y.N.; Kwon, Y.M.; Kim, M.C.; Kang, S.M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int. J. Mol. Med. 2014, 34, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Choi, H.J.; Song, H.H.; Hong, E.H.; Lee, B.R.; Oh, S.R.; Choi, K.; Yeo, S.G.; Lee, Y.P.; Cho, S.; et al. Antiviral activity of ginsenosides against coxsackievirus B3, enterovirus 71, and human rhinovirus 3. J. Ginseng Res. 2014, 38, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Zhang, G.; Tang, B.; Liu, Y.; Fu, X.; Zhang, X. Promising Anti-influenza Properties of Active Constituent of Withania somnifera Ayurvedic Herb in Targeting Neuraminidase of H1N1 Influenza: Computational Study. Cell Biochem. Biophys. 2015, 72, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. V Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.R.; Yang, C.S. Protective roles of ginseng against bacterial infection. Microb. Cell 2018, 5, 472–481. [Google Scholar] [CrossRef]
- Kim, G.; Kim, T.H.; Kang, M.J.; Choi, J.A.; Pack, D.Y.; Lee, I.R.; Kim, M.G.; Han, S.S.; Kim, B.Y.; Oh, S.M.; et al. Inhibitory effect of withaferin A on Helicobacter pylori-induced IL-8 production and NF-κB activation in gastric epithelial cells. Mol. Med. Rep. 2016, 13, 967–972. [Google Scholar] [CrossRef]
- Owais, M.; Sharad, K.S.; Shehbaz, A.; Saleemuddin, M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine 2005, 12, 229–235. [Google Scholar] [CrossRef]
- Arora, S.; Dhillon, S.; Rani, G.; Nagpal, A. The in vitro antibacterial/synergistic activities of Withania somnifera extracts. Fitoterapia 2004, 75, 385–388. [Google Scholar] [CrossRef]
- Sundaram, S.; Dwivedi, P.; Purwar, S. In vitro Evaluation of Antibacterial Activities of Crude Extracts of Withania somnifera (Ashwagandha) to Bacterial Pathogens. Asian J. Biotechnol. 2011, 3, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Eun, K.P.; Uhm, C.S.; Chung, M.S.; Kyung, H.K. Inhibition of Helicobacter pylori adhesion to human gastric adenocarcinoma epithelial cells by acidic polysaccharides from Artemisia capillaris and Panax ginseng. Planta Med. 2004, 70, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Choi, S.Y.; Park, S.J.; Paek, N.S.; Kim, S.S. Anti-Helicobacter Pylori effect of fermented ginseng extracts with Lactobacillus plantarum MG 208. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 53–56. [Google Scholar] [CrossRef]
- Park, S.; Yeo, M.; Jin, J.H.; Lee, K.M.; Jung, J.Y.; Choue, R.; Sung, W.C.; Hahm, K.B. Rescue of Helicobacter pylori—Induced cytotoxicity by red ginseng. Dig. Dis. Sci. 2005, 50, 1218–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jee, H.-S.; Chang, K.-H.; Moon, S.-H.; Park, S.-H.; Paik, H.-D. Anti-Helicobacter pylori, Cytotoxic, and Anti-inflammatory Activities of White Ginseng Extract. Food Sci. Biotechnol. 2008, 17, 1106–1109. [Google Scholar]
- Wu, H.; Lee, B.; Yang, L.; Wang, H.; Givskov, M.; Molin, S.; Høiby, N.; Song, Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med. Microbiol. 2011, 62, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Moser, C.; Wu, H.; Faber, V.; Kharazmi, A.; Høiby, N. Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection. J. Cyst. Fibros. 2003, 2, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.S.; Bae, K.G.; Jung, I.S.; Kim, C.H.; Yun, Y.S.; Song, J.Y. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J. Infect. 2002, 45, 32–38. [Google Scholar] [CrossRef]
- Ahn, J.-Y.; Choi, I.-S.; Shim, J.-Y.; Yun, E.-K.; Yun, Y.-S.; Jeong, G.; Song, J.-Y. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur. J. Immunol. 2006, 36, 37–45. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, D.G. The combination effect of Korean red ginseng saponins with kanamycin and cefotaxime against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2008, 31, 1614–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, P.; Yao, Y.; Yang, X.S.; Feng, J.; Ren, G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017, 41, 180–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Shim, J.S.; Lee, J.S.; Kim, M.K.; Chung, M.S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006, 341, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, S.E.; Huh, J.; Han, Y.H.; Lee, M.J. Antibacterial and antioxidative activity of roasted coffee and red ginseng mixture extracts. J. Korean Soc. Food Sci. Nutr. 2012, 41, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Pseudomonas aeruginosa Infection | HAI | CDC. Available online: https://www.cdc.gov/hai/organisms/pseudomonas.html (accessed on 29 January 2021).
- Song, Z.; Kharazmi, A.; Wu, H.; Faber, V.; Moser, C.; Johansen, H.K.; Rygaard, J.; Høiby, N. Effects of ginseng treatment on neutrophil chemiluminescence and immunoglobulin G subclasses in a rat model of chronic Pseudomonas aeruginosa pneumonia. Clin. Diagn. Lab. Immunol. 1998, 5, 882–887. [Google Scholar] [CrossRef]
- Alipour, M.; Omri, A.; Suntres, Z.E. Ginseng aqueous extract attenuates the production of virulence factors, stimulates twitching and adhesion, and eradicates biofilms of Pseudomonas aeruginosa. Can. J. Physiol. Pharmcol. 2011, 89, 419–427. [Google Scholar] [CrossRef]
- Song, Z.; Kong, K.F.; Wu, H.; Maricic, N.; Ramalingam, B.; Priestap, H.; Schneper, L.; Quirke, J.M.E.; Høiby, N.; Mathee, K. Panax ginseng has anti-infective activity against opportunistic pathogen Pseudomonas aeruginosa by inhibiting quorum sensing, a bacterial communication process critical for establishing infection. Phytomedicine 2010, 17, 1040–1046. [Google Scholar] [CrossRef] [Green Version]
- Horsley, A. Book review: Hodson and Geddes’ Cystic Fibrosis. Breathe 2016, 12, 91–92. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, D.M.; Wozniak, D.J. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005, 56, 309–322. [Google Scholar] [CrossRef]
- Song, Z.; Johansen, H.K.; Faber, V.; Moser, C.; Kharazmi, A.; Rygaard, J.; Høiby, N. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob. Agents Chemother. 1997, 41, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.O.; Lee, S.; Kim, S.J.; Rhee, D.K. Korean Red Ginseng enhances pneumococcal Δpep27 vaccine efficacy by inhibiting reactive oxygen species production. J. Ginseng Res. 2019, 43, 218–225. [Google Scholar] [CrossRef]
- Ahmed, A.B.M. Microbial toxinology for safer drug industry. J. Pharm. Care Health Syst. 2016, 03, 4. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Luong, T.T.; Lee, S.Y.; Kim, G.L.; Kwon, H.; Lee, H.G.; Park, C.K.; Rhee, D.K. Panax ginseng aqueous extract prevents pneumococcal sepsis in vivo by potentiating cell survival and diminishing inflammation. Phytomedicine 2015, 22, 1055–1061. [Google Scholar] [CrossRef]
- Ha, K.C.; Kim, M.G.; Oh, M.R.; Choi, E.K.; Back, H.I.; Kim, S.Y.; Park, E.O.; Kwon, D.Y.; Yang, H.J.; Kim, M.J.; et al. A placebo-controlled trial of Korean red ginseng extract for preventing Influenza-like illness in healthy adults. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElhaney, J.E.; Simor, A.E.; McNeil, S.; Predy, G.N. Efficacy and Safety of CVT-E002, a Proprietary Extract of Panax quinquefolius in the Prevention of Respiratory Infections in Influenza-Vaccinated Community-Dwelling Adults: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Trial. Influenza Res. Treat. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Kuhle, S.; Seida, J.K.; Durec, T. North American (Panax quinquefolius) and Asian ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: A systematic review. Evid.-Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Vohra, S.; Johnston, B.C.; Laycock, K.L.; Midodzi, W.K.; Dhunnoo, I.; Harris, E.; Baydala, L. Safety and tolerability of north american ginseng extract in the treatment of pediatric upper respiratory tract infection: A phase II randomized, controlled trial of 2 dosing schedules. Pediatrics 2008, 122, e402–e410. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, A.L.; Di, Y.M.; Shergis, J.L.; Chen, Y.; Guo, X.; Wen, Z.; Thien, F.; Worsnop, C.; Lin, L.; et al. Panax ginseng therapy for chronic obstructive pulmonary disease: A clinical trial protocol and pilot study. Chin. Med. 2014, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- High, K.P.; Case, D.; Hurd, D.; Powell, B.; Lesser, G.; Falsey, A.R.; Siegel, R.; Metzner-Sadurski, J.; Krauss, J.C.; Chinnasami, B.; et al. A Randomized, Controlled Trial of Panax quinquefolius Extract (CVT-E002) to Reduce Respiratory Infection in Patients With Chronic Lymphocytic Leukemia. J. Support. Oncol. 2012, 10, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.H.; Park, S.H.; Choi, E.K.; Jung, S.J.; Pyo, M.K.; Chae, S.W. A randomized, double-blind, placebo-controlled pilot study to assess the effects of protopanaxadiol saponin–enriched ginseng extract and pectinase-processed ginseng extract on the prevention of acute respiratory illness in healthy people. J. Ginseng Res. 2020, 44, 697–703. [Google Scholar] [CrossRef]
- Wang, M.; Guilbert, L.J.; Ling, L.; Li, J.; Wu, Y.; Xu, S.; Pang, P.; Shan, J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J. Pharm. Pharmacol. 2001, 53, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, E. Panax ginseng: An Overview of the Clinical Evidence. J. Ginseng Res. 2010, 34, 259–263. [Google Scholar] [CrossRef] [Green Version]






| Ginseng Extracts and Compounds | Respiratory Viruses | Study Type | Observations | Conclusions | Reference |
|---|---|---|---|---|---|
| seven compounds- mainly belonging to P. ginseng | Coronavirus | Glide docking program was utilized for molecular docking | Floralginsenoside B, which is extracted from Panax ginseng, indicated a docking score of −8.61 Kcal/mole and showed high binding affinity by interacting with active pocket residues of 6M0J mainly through hydrogen bonds with Gln474, Cys 480, Gly 482, Glu465, and Asp467 than other compounds against the SARS-CoV-2 Spike RBD. | The extracts and essential oils of Panax ginseng could be introduced as promising COVID-19 inhibitors. | [132] |
| Withania somnifera (Indian ginseng) | Coronavirus | Molecular docking and dynamics studies | Two different protein targets of SARS-CoV-2, namely NSP15 endoribonuclease and receptor binding domain of prefusion spike protein from SARS-CoV-2, were targeted. Molecular docking studies suggested Withanoside X and Quercetin glucoside from W. somnifera have favorable interactions at the binding site of selected proteins, that is, 6W01 and 6M0J. | Based on proven therapeutic potential against n-CoV-2 proteins, Indian ginseng could be one of the alternatives as an antiviral agent in the treatment of COVID-19. | [133] |
| Panax ginseng and Schizonepeta tenuifolia | SARS-CoV-2 and Infuenza A viruses. | In-vitro, Cells and cell culture, plasmid transfection and virus assembly, Cytotoxicity assays, Virus infection and drug inhibition assays | RDS contains broad-spectrum antiviral activity, blocking the infection of SARS-CoV, SARS-CoV-2, and Infuenza A viruses. | RDS may broadly inhibit the infection of respiratory viruses such as SARS-CoV, SARS-CoV-2, and Infuenza A. | [134] |
| Ginseng stem-leaf saponins (GSLS) in combination with selenium | Newcastle disease virus and infectious bronchitis virus | Female yellow chickens | In-vitro, Hemagglutination inhibition test, Immunohistochemical staining for IgG+, IgA+ and IgM+ cells, sIgA assay, RT-qPCR, Transcriptome analysis. | Enhanced antibody responses in GSLS-Se group may be attributed to the immunomodulatory effects of GSLS-Se on the immune-related gene profile expressed in the immunocompetent cells of the HGs. | [84] |
| Ginseng stem-and-leaf saponin (GSLS) | Newcastle disease virus | White layer chickens | Experiment design, Hemagglutination inhibition test, Immunohistochemical staining for IgG+, IgA+ and IgM+ cells, sIgA assay, RT-qPCR, Transcriptome analysis. | GSLS could be a useful oral adjuvant to improve vaccine immunization in chickens. | [135] |
| Extract of Korean red ginseng (RG) | Influenza A virus | In vitro and In vivo mice model | Polysaccharide fraction was most effective in reducing the accumulation of (TNF-α)/(iNOS)-producing dendritic cells (tip DCs) in the mouse lungs. | Polysaccharides of RG have a pronounced beneficial effect on the symptoms of influenza virus infection. | [136] |
| Extract of Korean red ginseng | H1N1 and H3N2 influenza viruses | In vitro, Naive mice model | Red ginseng extract showed significantly enhanced protection, lower levels of lung viral titers and interleukin-6, but higher levels of interferon-γ compared with control mice having virus infections without red ginseng extract. | Intake of ginseng extract will have beneficial effects on preventing lethal infection with newly emerging influenza viruses. | [137] |
| Panax ginseng polysaccharide (PGP) | H1N1 (A/PR/8/34) and H3N2 (A/Philippines/82) influenza viruses | In vitro, mice study | PGP solution showed moderately enhanced survival rates and lower levels of lung viral titers and the inflammatory cytokine (IL-6). | This study demonstrated that PGP can be used as a remedy against influenza viral infection. | [138] |
| Black ginseng (BG) and red ginseng (RG) | A(H1N1) pdm09 (A/California/04/2009) virus. | In vitro, mice study | BG displayed a 100% survival rate against infection, while mice treated with RG had a 50% survival rate. | BG may be useful as an alternative antiviral adjuvant to modulate immune responses to influenza A virus. | [139] |
| Fermented ginseng extracts | Different strains of influenza viruses, H1N1, H3N2, H5N1, and H7N9. | Different genetic backgrounds of mice and in the deficient conditions of key adaptive immune components (CD4, CD8, B cell, MHCII) | In vitro cell culture experiments showed moderate virus-neutralizing activity by fermented ginseng extract, probably by inhibiting hemagglutination and neuraminidase activity. | Fermented ginseng extracts might provide a means to treat influenza disease regardless of virus strains. | [79] |
| Red ginseng extract (RGE) | influenza A virus | In vivo and in vitro, mice model | RGE was found to improve survival of human lung epithelial cells upon influenza virus infection. Also, RGE treatment reduced the expression of pro-inflammatory genes (IL-6, IL-8). | RGE might have the potential beneficial effects on preventing influenza A virus infections via its multiple immunomodulatory functions. | [90] |
| Ginseng extract and ginsenosides | Influenza A virus | In vivo and in vitro, mice model | Ginsenosides protected the animals from lethal 2009 pandemic H1N1 infection and lowered viral titers in animal lungs. | Ginsenosides are promising candidates for the development of antiviral drugs for influenza viruses. | [78] |
| Ginseng | Respiratory syncytial virus | BALB/c mice after RSV infection | Ginseng-treated mice that were non-immunized or previously immunized with FI-RSV showed improved protection against RSV challenge compared with control mice without ginseng treatment. | Ginseng can modulate host immune responses to FI-RSV immunization and RSV infection, resulting in protective effects against pulmonary inflammatory disease. | [140] |
| Panax Korean red ginseng extract (KRGE) | Respiratory syncytial virus | In vitro and in vivo | KRGE improved the survival of human lung epithelial cells against RSV infection and inhibited RSV replication. | Results suggested that KRGE has antiviral activity against RSV infection | [141] |
| Red ginseng extract (RGE) | RSV | In vitro cell culture and in vivo mouse models | RGE treatment improved lung viral clearance and enhanced the production IFN-γ in bronchoalveolar lavage cells upon RSV infection of mice. | Ginseng has protective effects against RSV infection through multiple mechanisms. | [23] |
| Seven ginsenosides | Human rhinovirus | Assays for antiviral activity and cytotoxicity were carried out by the sulforhodamine B method using the cytopathic effect (CPE) reduction assay. | The antiviral assays demonstrated that, of the seven ginsenosides, the PT-type ginsenosides (Re, Rf, and Rg2) possess significant antiviral activities against CVB3 and HRV3 at a concentration of 100 μg/mL. Only ginsenoside Rg2 showed significant anti-EV71 activity with no cytotoxicity to cells at 100 μg/mL | Ginsenosides Re, Rf, and Rg2 have the potential to be effective in the treatment of CVB3, EV71, and HRV3 infection. | [142] |
| Withania somnifera (Indian ginseng) | H1N1 Influenza virus | In silico study | High binding affinity of the WA toward NA and revealed several interesting molecular interactions with the residues which are catalytically important during molecular dynamic simulations. | Several interesting molecular interactions with the residues which are catalytically important during molecular dynamic simulations. | [143] |
| Ginseng Extracts and Compounds | Microbe | Study Type | Observations | Conclusions | Reference |
|---|---|---|---|---|---|
| Withaferin A (WA), a withanolide purified from Withania somnifera | H. pylori | In vitro study | WA inhibits H. pylori-induced IL-8 production in gastric epithelial cells. | WA does not influence H. pylori-induced ROS production or any associated signaling. | [147] |
| Withania somnifera (Indian ginseng), Both aqueous as well as alcoholic extracts of the plant (root as well as leaves) | Pathogenic bacteria | In vitro study | Inhibitory activity against a spectrum of bacteria. | Increased survival rate as well as decreased bacterial load. | [148] |
| Withania somnifera (Indian ginseng) extracts | Salmonella typhimurium and Escherichia coli. | In vitro study | Methanol and hexane extracts of both leaves and roots were found to have potent antibacterial activity. | A synergistic increase in the antibacterial effect of Tibrim was noticed when MIC of Tibrim was supplemented with these extracts. | [149] |
| Extracts of Withania somnifera (Indian ginseng) | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis | In vitro study | Polar solvents had higher antibacterial property in comparison with the nonpolar solvents; higher MIC values were obtained for both gram-positive bacteria S. aureus, B. subtilis and gram-negative bacteria, E. coli and P. aeruginosa, with polar extract. | Antimicrobial activity of crude extract of W. somnifera was shown to validate the use of traditional medicinal herbal medicine and results of this study tend to give credence to the common use of W. somnifera plant. | [150] |
| P. ginseng polysaccharides | H. pylori | hemagglutination and enzyme-linked glycosorbent assays | Acidic carbohydrates may play an important role in the inhibitory activity on H. pylori adhesion to host cells. | Bacterial binding was inhibited more effectively by P. ginseng polysaccharides | [151] |
| Fermented ginseng extracts | H. pylori | Formation of clear zones, measurement of urease activity and cell adhesion activity in vitro. | Anti-H. pylori activity, including anti-bacterial, anti-adhesion, and urease inhibition effects. | Fermented ginseng extract containing L.plantarum MG 208 could prove to be useful as a functional diet for the protection of the gastric environment against H. pylori. | [152] |
| Red ginseng extracts (RGE) | H. pylori | Analysis of cell viability (trypan blue dye exclusion assay, DNA fragmentation assay (comet assay) Measurement of cytokine level, cell signaling (in vitro) | RGE decreased H. pylori-stimulated IL-8 gene expression, which resulted from the transcriptional regression of NF-κB. | RGE showed significant gastroprotective effects against H. pylori-associated gastric mucosal cell damage, suggesting that red ginseng could be used as a medicinal phytonutrient against H. pylori infection. | [153] |
| White ginseng extract (WGE) | H. pylori | Disc diffusion assay | The zone of inhibition due to WGE increased significantly with increasing dosage. WGE exhibited an inhibitory effect on cell growth at 2.0 mg/mL for all tumor cell lines. | The potential of WGE to be used as a health-promoting substance. | [154] |
| Ginseng aqueous extract | Pseudomonas aeruginosa | P. aeruginosa biofilms were further investigated in vitro and in vivo. | Oral administration of ginseng extracts in mice promoted phagocytosis of P. aeruginosa PAO1 by airway phagocytes but did not affect phagocytosis of a PAO1-film mutant. | Ginseng treatment may help to eradicate the biofilm-associated chronic infections caused by P. aeruginosa. | [155] |
| Saline extract of ginseng | Pseudomonas aeruginosa | Cytokine modulating effect in a mouse model of P. aeruginosa lung infection. | Th1-like immune response in the mice with P. aeruginosa lung infection after 7 days of ginseng treatment. | Th1 response might benefit the host with P. aeruginosa lung infection and ginseng treatment might be a promising alternative measure for the treatment of chronic P. aeruginosa lung infection in CF patients. | [156] |
| Polysaccharide (PS) isolated from Panax ginseng | Staphylococcus aureus | In vitro assays for the activity measurement of PS, NO production test with Greiss reagent, in vivo anti-septicemic activity was assessed by using C57BL/6J mice. | Polysaccharide showed anti-septic effects, Ginsan enhanced pro-inflammatory abilities (NO, pro-inflammatory cytokine production, phagocytic activity of macrophages). Ginsan modulated TLR pathway. | PS from Panax ginseng possess a potent anti-septicemic activity by stimulating macrophage and potential as an immunomodulator against sepsis caused by Staphylococcus aureus. | [157] |
| Polysaccharide (PS) isolated from Panax ginseng | Staphylococcus aureus | In vitro study | Proinflammatory cytokines, such as TNF-alpha, IL-1beta, IL-6, IFN-gamma, IL-12, and IL-18, were markedly down-regulated in ginsan-treated mice compared with those of control-infected mice. | Antiseptic activity of ginsan can be attributed to enhanced bacterial clearance, and reduced proinflammatory cytokines via the TLR signaling pathway. | [158] |
| Korean red ginseng | Staphylococcus aureus | Fluorescent marker calcein from negatively charged PC/PG (1: 1, w/w) liposomes | Ginsenosides may exert antibacterial activity by disrupting the cell membrane | Synergistic or additive effects between the ginsenosides and antibiotics tested | [159] |
| Crude saponins extracted from the Panax quinquefolius | Fusobacterium nucleatum, Clostridium perfringens, and Porphyromonas gingivalis | Determination of MIC, cell integrity | HTS, HTS-3, and HTS-4 were effective at inhibiting the growth of F. nucleatum, C. perfringens, and P. gingivalis. | Less polar ginsenoside-enriched fraction from heat transformation can be used as an antibacterial agent to control halitosis. | [160] |
| Acidic polysaccharide from P. ginseng, PG-F2 | P. gingivalis | Determination of MIC | Anti-adhesive activity and anti-hemagglutination. | PG-F2 may exert a selective antiadhesive effect against pathogenic bacteria, while having no effects on beneficial and commensal bacteria. | [161] |
| A mixture of roasted coffee and red ginseng | Pseudomonas aeruginosa and S. Typhimurium | Classical paper disc method | DPPH scavenging activity decreased when red ginseng extract composed of more than 70% of the total extract. | Antibacterial activity shown. | [162] |
| Participants | Interventions | Comparisons | Outcomes | Study Design | References |
|---|---|---|---|---|---|
| 100 participants Age group 30–70 years | KRGE Nine capsules per day for three months | A Placebo-Controlled Trial of Korean Red Ginseng Extract to Prevent Acute Respiratory Illness in Healthy Subjects | Reduced Influenza-such as illness (ILI) incidence | Interventional (Clinical Trial) | [173] |
| 43 participants ≥ 65 years of age | 2 capsules/day of either COLD-fX or placebo (200 mg/capsule) for 4 months. | COLD-fX or placebo | Ingestion of COLD-fX by immunocompetent seniors during an early “cold and flu” season reduced the relative risk and duration of respiratory symptoms by 48% and 55%, respectively. | A randomized, double-blind, placebo-controlled trial. | [117] |
| 783 community-dwelling adults. | Adults were randomized to receive placebo, 400 mg, or 800 mg treatment. | CVT-E002 (a proprietary) A double-blind, placebo-controlled trial. | CVT-E002 (a proprietary extract) can be safely used by similar groups and may prevent URI symptoms, Jackson-confirmed. | A multicenter, randomized, double-blind, placebo-controlled trial. | [174] |
| 747 participants, more than equal to 18 years. | North American (Panax quinquefolius) or Asian ginseng (Panax ginseng) root extract or placebo or no treatment in healthy adults were included. | (P. quinquefolius or P. ginseng) root extract or placebo | Significantly reduced the total number of common colds by 25% compared with placebo. Tendency towards lower incidence of at least one common cold or other acute respiratory infection (ARI) in the ginseng group compared with the placebo group. | Randomized controlled trials or controlled clinical trials. | [175] |
| Eighty-nine (2000) and 109 (2000–2001) enrolled participants, average age 81 and 83.5, respectively; 74% women. | Oral twice-daily administration of a proprietary ginseng extract, CVT-E002, 200 mg, or placebo. | Proprietary extract of American ginseng, CVT-E002, with placebo in preventing acute respiratory illness (ARI) | CVT-E002 was shown to be safe, well tolerated, and potentially useful for preventing ARI due to influenza and RSV. | Two randomized, double-blind, placebo-controlled trials | [115] |
| 323 subjects 18–65 years of age with a history of at least 2 colds in the previous year were recruited from Edmonton’s general population. | Two capsules per day of either the North American ginseng extract or a placebo for 4 months. | North American ginseng extract or a placebo. | Moderate dose over 4 months reduced the mean number of colds per person. | A randomized, double-blind, placebo-controlled | [116] |
| 75 subjects, children 3 to 12 years of age. | Two dosing schedules of American ginseng extract during the winter months | American ginseng extract or a placebo. | Standard doses of ginseng were well tolerated and merit additional evaluation concerning pediatric upper respiratory tract infection treatment. | A randomized, double-blind dose-finding three-arm trial | [176] |
| 14 participants (57–73 years old) with moderate to very severe COPD. | 200 mg twice daily for four weeks) and then followed-up for an additional 4 weeks for a total of 10 weeks. | P. ginseng or a placebo | COPD exacerbations or adverse events | A randomized, double-blind, placebo-controlled clinical trial | [177] |
| 500 children aged 3–11 | Either COLD-FX or placebo for 3 days. | COLD-FX or a placebo | No results posted | A randomized, double-blind, placebo-controlled clinical trial. | ClinicalTrials.gov identifier (NCT number): NCT00965822 |
| 200 participants aged 12–75 years. | 200 mg twice daily for 4 weeks. Other Name: CVT-E002 | COLD-FX or a placebo | No results posted | A randomized, double-blind, placebo-controlled clinical trial. | ClinicalTrials.gov identifier (NCT number): NCT00726401 |
| 293 subjects with early-stage Chronic Lymphocytic Leukemia (CLL) | Oral extract for 3 months twice a day | COLD-FX or a placebo | Reduced rates of moderate-severe ARI and significantly less sore throat, enhanced antibody | A double-blind, placebo-controlled, randomized trial. | [178] |
| 227 volunteers | Daily oral capsule doses of either placebo (113) or 100 mg of standardized ginseng extract Ginsana G 115 (114) for 12 weeks. | Ginsana G 115 (114) Or a placebo | Natural killer (NK) activity levels two-fold in the G115 group; can protect against common cold and influenza. | Multicenter, two-arm, randomized, placebo-controlled, double-blind | [81] |
| 100 volunteers | Three times/day, 9 capsules/day, (3 g/day) for 12 weeks. | Korean Red Ginseng (KRG) or a placebo | May be useful in protecting subjects from contracting ARI and may decrease the duration and scores of ARI symptoms. | A randomized, double-blinded, placebo-controlled | [122] |
| 45 healthy applicants aged 39–65 years | Six capules/day, 500 mg/capsule for 8 weeks. | GS-3K8 (ultra-filtered red ginseng extract) 2. GINst15 (hydrolyzed ginseng extract) or a placebo | GS-3K8 and GINST appear to have a positive tendency toward preventing ARI development and reducing the symptom duration. | A randomized, double-blind, placebo-controlled, pilot study at a single center. | [179] |
| 328 subjects Age ≥ 18 Years | Sachet’s granules, Oral route at day 7 | Drug: Jing Fang Bai Du san Drug: Placebo Drug: Ying Qiao san | Jing Fang Bai Du San relieved and effectively cleared up the pathogenic cold. Ying Qiao san effectively cleared up the pathogenic heat. | A randomized, double-blind, placebo-controlled | ClinicalTrials.gov identifier (NCT number): NCT00887172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsayari, A.; Muhsinah, A.B.; Almaghaslah, D.; Annadurai, S.; Wahab, S. Pharmacological Efficacy of Ginseng against Respiratory Tract Infections. Molecules 2021, 26, 4095. https://doi.org/10.3390/molecules26134095
Alsayari A, Muhsinah AB, Almaghaslah D, Annadurai S, Wahab S. Pharmacological Efficacy of Ginseng against Respiratory Tract Infections. Molecules. 2021; 26(13):4095. https://doi.org/10.3390/molecules26134095
Chicago/Turabian StyleAlsayari, Abdulrhman, Abdullatif Bin Muhsinah, Dalia Almaghaslah, Sivakumar Annadurai, and Shadma Wahab. 2021. "Pharmacological Efficacy of Ginseng against Respiratory Tract Infections" Molecules 26, no. 13: 4095. https://doi.org/10.3390/molecules26134095